238198
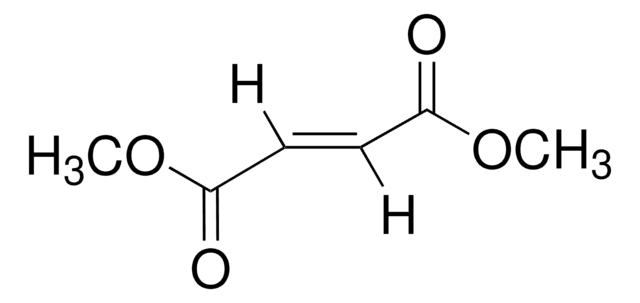
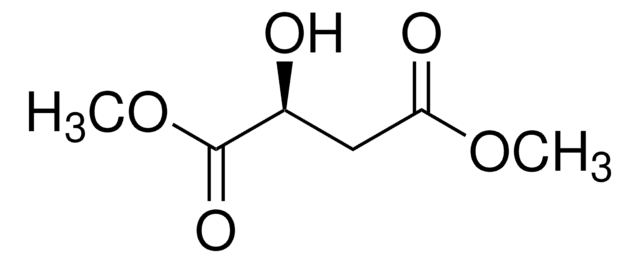
Dimethyl maleate
96%
동의어(들):
(2Z)-2-Butenedioic acid dimethyl ester, (Z)-2-Butenedioic acid dimethyl ester, (Z)-Dimethyl 2-butenedioate, Dimethyl (Z)-but-2-enedioate
About This Item
추천 제품
Quality Level
분석
96%
양식
liquid
불순물
≤4% dimethyl fumarate
refractive index
n20/D 1.441 (lit.)
bp
204-205 °C (lit.)
solubility
water: soluble 77.9 g/L at 20 °C
density
1.152 g/mL at 25 °C (lit.)
작용기
ester
SMILES string
[H]\C(=C(/[H])C(=O)OC)C(=O)OC
InChI
1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3-
InChI key
LDCRTTXIJACKKU-ARJAWSKDSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Dimethyl maleate (DMM) is a reactive dienophile and undergoes ultrasonic irradiation promoted Diels-Alder reaction with substituted furans. Mesoporous siliceous SBA-15-supported Cu catalyzed gas phase hydrogenolysis of DMM to 1,4-butanediol (BDO) has been reported. Aluminium chloride has been reported to accelerate the Diels-Alder reaction of DMM and anthracene. DMM can be synthesized by the esterification of maleic anhydride with sulfuric acid and methanol.
애플리케이션
- Dissociation of bovine 6S procarboxypeptidase A by reversible condensation with 2,3-dimethyl maleic anhydride: application to the partial characterization of subunit III.: This study explores the dissociation of bovine procarboxypeptidase A using 2,3-dimethyl maleic anhydride, highlighting its applications in the partial characterization of enzyme subunits. The research demonstrates the potential of dimethyl maleate derivatives in protein chemistry and enzyme structure studies. (Puigserver and Desnuelle, 1975).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1 - STOT RE 2 Dermal - STOT SE 3
표적 기관
Respiratory system, Skin
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point (°F)
203.0 °F - closed cup
Flash Point (°C)
95 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
문서
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.