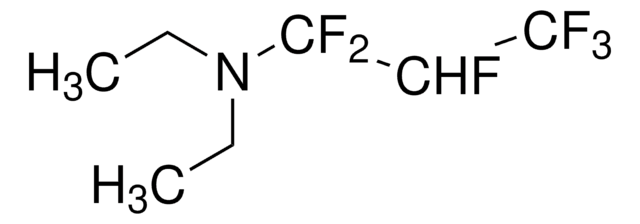
추천 제품
분석
95%
양식
liquid
bp
30-32 °C/3 mmHg (lit.)
density
1.22 g/mL at 25 °C (lit.)
작용기
amine
저장 온도
2-8°C
SMILES string
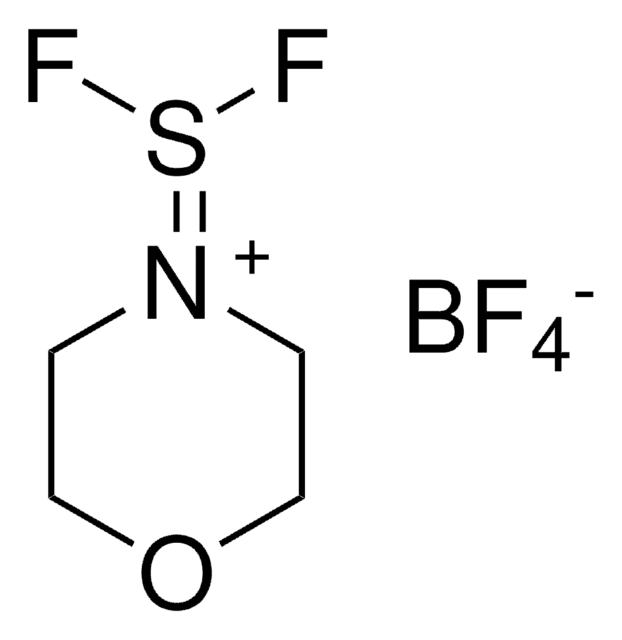
CCN(CC)S(F)(F)F
InChI
1S/C4H10F3NS/c1-3-8(4-2)9(5,6)7/h3-4H2,1-2H3
InChI key
CSJLBAMHHLJAAS-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Diethylamino sulfur trifluoride (DAST) is a fluorinating reagent used in the synthesis of fluorinated compounds and ring-opening reactions.
애플리케이션
- Fluorinating agent: reaction with alcohols and carbonyl compounds, Review
- Review on nucleophilic fluorination.
- Catalyst for Friedel-Crafts allylation using tertiary cyclopropyl silyl ethers and the rearrangement of homoallylic alcohols to unsaturated aldehydes.
- Early introduction of a fluoromethyl group stabilizes the epoxide during further manipulations in the synthesis of 26-fluoro-epothilone.
- Fluorinating agent for a variety of compounds, including thioethers, alkenols, and cyanohydrins.
- Reagent for gem difluorination of ketopipecolinic acids.
교체됨
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Self-react. D - Skin Corr. 1A
보충제 위험성
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point (°F)
73.4 °F
Flash Point (°C)
23 °C
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
A J Phillips et al.
Organic letters, 2(8), 1165-1168 (2000-05-11)
[formula: see text] A mild and highly efficient cyclization of beta-hydroxy amides to oxazolines is described using DAST and Deoxo-Fluor reagents. A one-pot protocol for the synthesis of oxazoles from beta-hydroxy amides is also presented.
P G Hultin et al.
Carbohydrate research, 322(1-2), 14-25 (2000-01-12)
We have made thioglycoside donors for the 4,6-dideoxy-L-lyxo-hexopyranosyl ('4-deoxy-L-rhamnosyl') and 4-deoxy-4-fluoro-L-rhamnosyl monosaccharide residues. The preparation of the deoxyfluororhamnose was not straightforward, and revealed some unexpected behavior of the diethylaminosulfur trifluoride (DAST) reagent. The new glycosyl donors were used to synthesize
Po-Chiao Lin et al.
The Journal of organic chemistry, 74(11), 4041-4048 (2009-05-16)
When saccharides bearing a sulfur, selenium, or oxygen substituent at the anomeric center and an unprotected hydroxyl group either at C-4 or C-6 were subjected to fluorination with DAST in dichloromethane, a regioselective migration of the anomeric substituent to the
Aurélien Bigot et al.
Organic letters, 13(2), 192-195 (2010-12-15)
The design of a new potent nonsteroidal ecdysone agonist led to the discovery of a diethylaminosulfur trifluoride (DAST)-mediated cyclization of α,α-disubstituted-α-acylaminoketones. The resulting fluorooxazolines can be ring-opened or selectively substituted by a range of nucleophiles to provide in high yields
Jindřich Karban et al.
Organic & biomolecular chemistry, 10(2), 394-403 (2011-11-16)
A complete series of eight 1,6:2,3- and 1,6:3,4-dianhydro-β-D-hexopyranoses were subjected to fluorination with DAST. The 1,6:3,4-dianhydropyranoses yielded solely products of skeletal rearrangement resulting from migration of the tetrahydropyran oxygen (educts of D-altro and D-talo configuration) or of the 1,6-anhydro bridge
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)