모든 사진(1)
About This Item
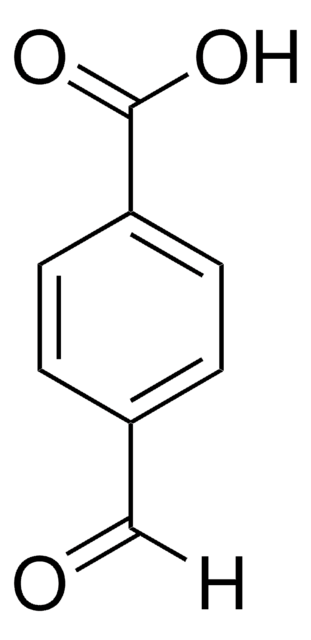
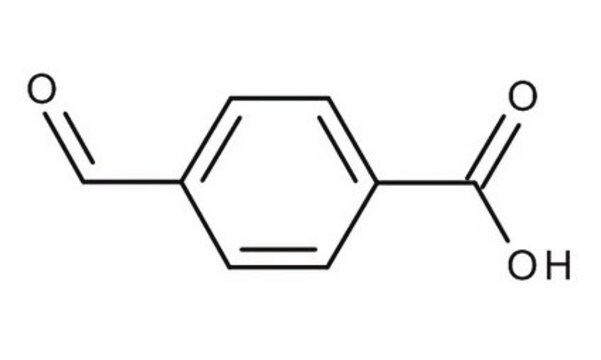
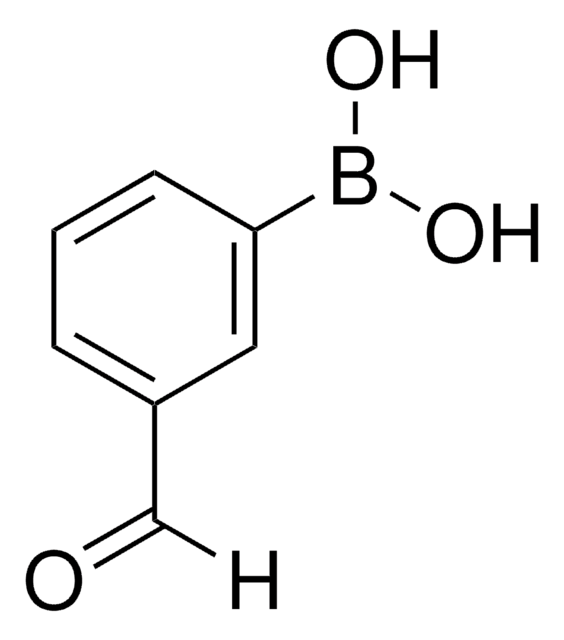
Linear Formula:
HO2CC6H4CHO
CAS Number:
Molecular Weight:
150.13
Beilstein:
2206413
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
97%
양식
powder
mp
173-175 °C (lit.)
solubility
methanol: soluble 100 mg/mL, clear to slightly hazy, colorless to very faintly brown(lit.)
작용기
aldehyde
carboxylic acid
SMILES string
[H]C(=O)c1cccc(c1)C(O)=O
InChI
1S/C8H6O3/c9-5-6-2-1-3-7(4-6)8(10)11/h1-5H,(H,10,11)
InChI key
UHDNUPHSDMOGCR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
3-Formylbenzoic acid is a polar aromatic aldehyde used in the synthesis of 3-hydroxymethylbenzoic acid via reduction.
애플리케이션
3-Formylbenzoic acid was used in the synthesis of:
- bicyclic cis-2-azetidinone derivatives via Ugi 4-centre 3-component reaction
- porphyrin capped with a steroidal superstructure bearing convergent hydroxy groups
- 3-[(4-amino-1,2-dihydro-1-oxo-2-phenyl-1,2,4-triazolo[4,3-a]quinoxalin-6-yl)amino]methylbenzoic acid
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Synthesis of Alicyclic-lactams via the Ugi Reaction on a Solid Support.
Gedey S, et al.
Letters in Organic Chemistry, 1(3), 215-220 (2004)
Suhman Chung et al.
Nature chemical biology, 5(6), 407-413 (2009-04-28)
The linking together of molecular fragments that bind to adjacent sites on an enzyme can lead to high-affinity inhibitors. Ideally, this strategy would use linkers that do not perturb the optimal binding geometries of the fragments and do not have
Vittoria Colotta et al.
Bioorganic & medicinal chemistry, 11(24), 5509-5518 (2003-12-04)
In previous papers (Colotta, V. et al. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 39. Colotta, V. et al. J. Med. Chem. 2000, 43, 1158) we reported the synthesis and binding affinity at bovine (b) A(1) and A(2A) and human
Synthesis, binding properties and self-functionalization of a steroid-capped porphyrin.
Richard P and Jeremy KM.
Journal of the Chemical Society. Chemical Communications, 8, 574-577 (1991)
Steven R Inglis et al.
Journal of medicinal chemistry, 52(19), 6097-6106 (2009-09-08)
Penicillin binding proteins (PBPs) catalyze steps in the biosynthesis of bacterial cell walls and are the targets for the beta-lactam antibiotics. Non-beta-lactam based antibiotics that target PBPs are of interest because bacteria have evolved resistance to the beta-lactam antibiotics. Boronic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.