220515
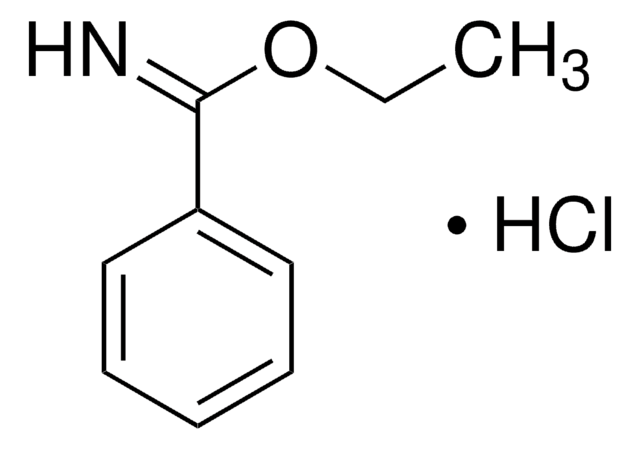
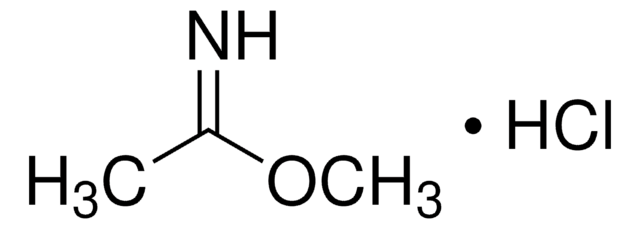
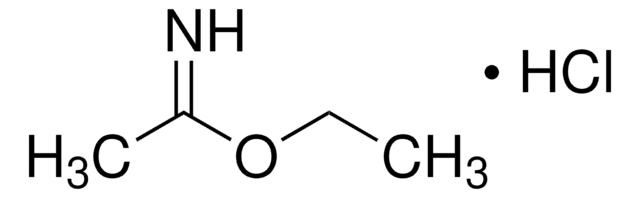
Methyl benzimidate hydrochloride
97%
동의어(들):
Benzimidoic acid methyl ester hydrochloride, Methyl benzenecarboximidate hydrochloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C6H5C(=NH)OCH3·HCl
CAS Number:
Molecular Weight:
171.62
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
105-107 °C (dec.) (lit.)
작용기
ether
phenyl
저장 온도
−20°C
SMILES string
Cl.COC(=N)c1ccccc1
InChI
1S/C8H9NO.ClH/c1-10-8(9)7-5-3-2-4-6-7;/h2-6,9H,1H3;1H
InChI key
HDJNHVNQRJMWSH-UHFFFAOYSA-N
애플리케이션
Methyl benzimidate hydrochloride was used:
- in the synthesis of chiral phenyldihydroimidazole derivative
- as imidating reagent to modify Lys residues of cyclic Lys-Gly-Asp peptide to afford acetimidate analogs
- in the synthesis of N-benzimidoyl-(1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine)
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
R M Scarborough et al.
The Journal of biological chemistry, 268(2), 1066-1073 (1993-01-15)
Members of the snake venon-derived, "disintegrin" peptide family containing the Arg-Gly-Asp (RGD) amino acid sequence are among the most potent inhibitors of the binding of adhesive proteins to platelet glycoprotein (GP) IIb-IIIa. However, GPIIb-IIIa antagonists containing the RGD sequence are
J Einsiedel et al.
Bioorganic & medicinal chemistry letters, 11(18), 2533-2536 (2001-09-11)
Conformationally restricted benzamide bioisosteres were investigated when the chiral phenyldihydroimidazole derivative 4e (FAUC 179) showed strong and highly selective dopamine D4 receptor binding (K(i)high=0.95nM). Mitogenesis experiments indicated partial agonist properties (42%). EPC syntheses of the target compounds of type 4
Tao Ji et al.
Chemical research in toxicology, 20(4), 701-708 (2007-03-27)
Thiobenzamide (TB) is hepatotoxic in rats causing centrolobular necrosis, steatosis, cholestasis, and hyperbilirubinemia. It serves as a model compound for a number of thiocarbonyl compounds that undergo oxidative bioactivation to chemically reactive metabolites. The hepatotoxicity of TB is strongly dependent
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.