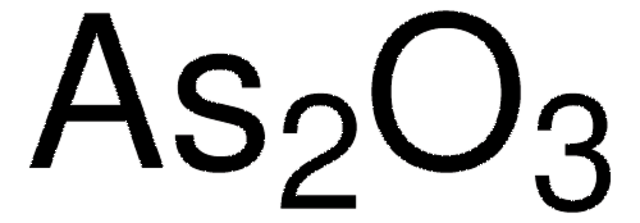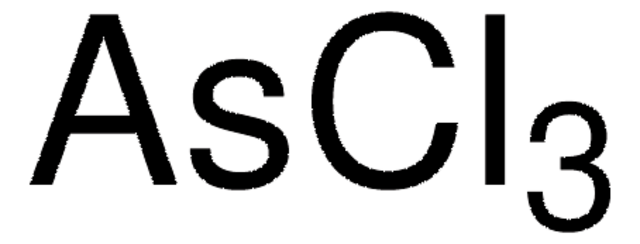추천 제품
Quality Level
분석
99.999% trace metals basis
저항도
33.3 μΩ-cm
bp
613 °C (lit.)
mp
817 °C (lit.)
density
5.727 g/mL at 25 °C (lit.)
SMILES string
[As]
InChI
1S/As
InChI key
RQNWIZPPADIBDY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Arsenic pieces may be used to prepare α-realgar (As4S4).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Repr. 1A - STOT RE 1 Oral
표적 기관
Respiratory organs,Cardio-vascular system,Gastro-intestinal system,Central nervous system,Nervous system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Valence and core-level binding energy shifts in realgar (As 4 S 4) and pararealgar (As 4 S 4) arsenic sulfides.
Bullen HA, et al.
Surface Science, 531(3), 319-328 (2003)
Claes Bergqvist et al.
Environmental pollution (Barking, Essex : 1987), 184, 540-546 (2013-11-05)
The toxicity of arsenic (As) in the environment is controlled by its concentration, availability and speciation. The aims of the study were to evaluate the accumulation and speciation of As in carrot, lettuce and spinach cultivated in soils with various
M Azizur Rahman et al.
Aquatic toxicology (Amsterdam, Netherlands), 146, 212-219 (2013-12-11)
Arsenic (As) is extremely toxic to living organisms at high concentration. In aquatic systems, As exists in different chemical forms. The two major inorganic As (iAs) species are As(V), which is thermodynamically stable in oxic waters, and As(III), which is
Lucia Cavalca et al.
Future microbiology, 8(6), 753-768 (2013-04-17)
Arsenic is present in many environments and is released by various natural processes and anthropogenic actions. Although arsenic is recognized to cause a wide range of adverse health effects in humans, diverse bacteria can metabolize it by detoxification and energy
Arsenic binding to proteins.
Shengwen Shen et al.
Chemical reviews, 113(10), 7769-7792 (2013-07-03)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 202657-25G | |
| 202657-5G | 4061838765130 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.