193801
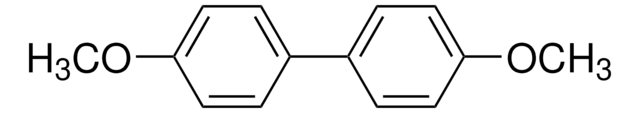
4,4′-Di-tert-butylbiphenyl
99%
동의어(들):
1-tert-Butyl-4-(4-tert-butylphenyl)benzene, 4,4′-Bis(1,1-dimethylethyl)-1,1′-biphenyl, 4,4′-Di-tert-butyl-1,1′-biphenyl, DBB, p,p′-Di-tert-butylbiphenyl
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
(CH3)3CC6H4C6H4C(CH3)3
CAS Number:
Molecular Weight:
266.42
Beilstein:
2095855
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
4,4′-Di-tert-butylbiphenyl along with lithium catalyzes:
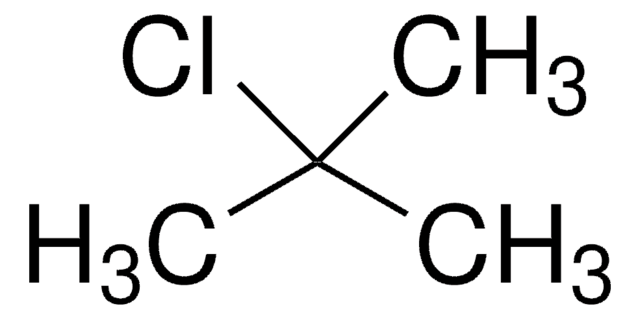
- reaction of chloromethyl ethyl ether and different carbonyl compounds to yield corresponding hydroxyethers
- reductive opening of N-phenylazetidine
애플리케이션
4,4′-Di-tert-butylbiphenyl was used in production of homoallylic amine derivatives. It was also used in the preparation of lithium di-tert-butylbiphenylide, a radical anion, superior to sodium or lithium naphthalenides for metalation reactions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
4, 4'-Di -tert -butylbiphenyl-catalysed reductive opening of azetidines with lithium: A direct preparation of 3, N-dilithioalkylamines.
Almena J, et al.
Tetrahedron, 50(19), 5775-5782 (1994)
4, 4′-Di-tert-butylbiphenyl-catalysed lithiation of chloromethyl ethyl ether: A barbier-type new and easy alternative to ethyl lithiomethyl ether.
Guijarro A and Yus M.
Tetrahedron Letters, 34(21), 3487-3490 (1993)
The Journal of Organic Chemistry, 55, 1528-1528 (1990)
C E Neipp et al.
The Journal of organic chemistry, 66(2), 531-537 (2001-06-30)
Lithiation of the N-2,4,6-triisopropylbenzenesulfonyl-2-pyrroline (16) and treatment of the resulting cyclic vinyllithium reagent with R2CuCNLi2 produced an acyclic vinyl organometallic species that, when treated with an electrophile (H2O or RX), gave the homoallylic sulfonamides 18a-k in 37-93% yields and in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.