추천 제품
분석
98%
mp
207-209 °C (lit.)
SMILES string
O=C1NNC(=O)N1c2ccccc2
InChI
1S/C8H7N3O2/c12-7-9-10-8(13)11(7)6-4-2-1-3-5-6/h1-5H,(H,9,12)(H,10,13)
InChI key
GOSUFRDROXZXLN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
4-Phenylurazole undergoes oxidation in the presence of NO2-N2O4 to yield 4-phenyl-1,2,4-trizoline-3,5-dione. It undergoes acetylation reaction with excess acetyl chloride in N,N-dimethylacetamide solution. It polymerizes in the presence of phosgene, terephthaloyl chloride and epichlorohydrin to yield insoluble polymer. It is precursor to the Diels-Alder trapping agent, 4-phenyl-1,2,4-triazoline-3,5-dione. This urazole was recently demonstrated in the traceless, chemoselective labeling of peptides and proteins through electrochemical tyrosine-click (e-Y-CLICK) chemistry.
관련 제품
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
A new method for the oxidation of 4-phenylurazole to 4-phenyltriazolinedione.
Mallakpour SE.
Journal of Chemical Education, 69(3), 238-238 (1992)
Bulletin of the Chemical Society of Japan, 61, 3915-3915 (1988)
Synthesis of aliphatic polyamides containing 4-phenylurazole linkages.
Mallakpour SE and Sheikholeslami B.
Polymer International, 48(1), 41-46 (1999)
Journal of the American Chemical Society, 109, 3730-3730 (1987)
Polycondensation Reaction of 4-(4'-N-1, 8-Naphthalimidophenyl)-1, 2, 4-triazolidine-3, 5-dione with Aliphatic Diacid Chlorides.
Mallakpour S and Rafiee Z.
Iranian Polymer Journal, 13, 225-234 (2004)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.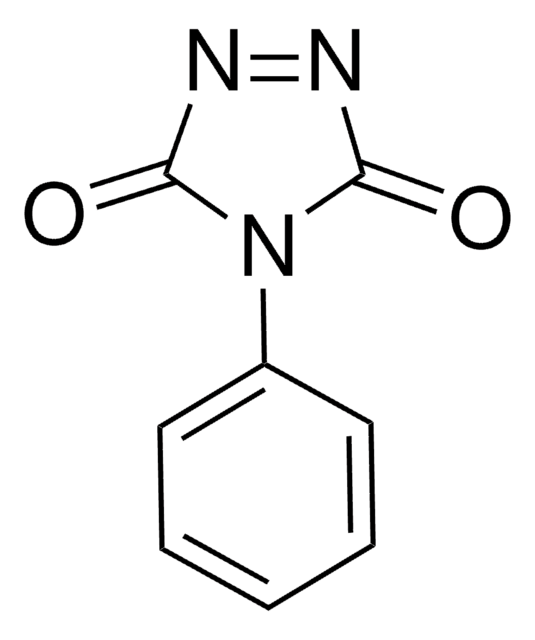



![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)
