18509
(+)-trans-Chrysanthemic acid
≥97.0% (GC)
동의어(들):
(1R,3R)-trans-2,2-Dimethyl-3-(2-methyl-1-propenyl)cyclopropane-1-carboxylic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C10H16O2
CAS Number:
Molecular Weight:
168.23
Beilstein:
4904351
EC Number:
MDL number:
UNSPSC 코드:
12352002
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
≥97.0% (GC)
광학 활성
[α]/D +14±1°, c = 2% in ethanol
refractive index
n20/D 1.477
작용기
carboxylic acid
SMILES string
C\C(C)=C/[C@@H]1[C@@H](C(O)=O)C1(C)C
InChI
1S/C10H16O2/c1-6(2)5-7-8(9(11)12)10(7,3)4/h5,7-8H,1-4H3,(H,11,12)/t7-,8+/m1/s1
InChI key
XLOPRKKSAJMMEW-SFYZADRCSA-N
일반 설명
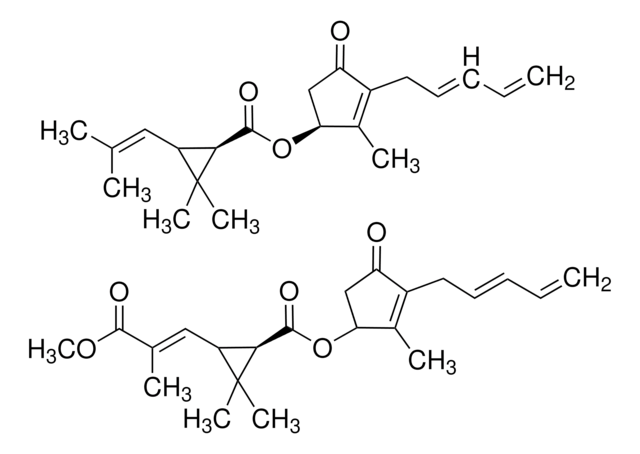
(+)-trans-Chrysanthemic acid is the acidic component of synthetic pyrethroid insecticides. It can be prepared from (+)-Δ3-carene.
애플리케이션
(+)-trans-Chrysanthemic acid may be used in the preparation of (+)-trans-pyrethric acid, a building block for rethrin II.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Wolfgang Bicker et al.
Journal of chromatography. A, 1035(1), 37-46 (2004-05-01)
This study reports on the direct HPLC stereoisomer separation of selected pyrethroic acids employing commercial cinchona alkaloid derived chiral stationary phases (CSPs). cis/trans-Chrysanthemic acid (cis/trans-CA), cis/trans-chrysanthemum dicarboxylic acid (cis/trans-CDCA), cis/trans-permethrinic acid (cis/trans-PA), and fenvaleric acid (FA) were resolved into the
[Evaluation of the toxicity of chrysanthemic acid and its derivatives].
A I Gurova et al.
Gigiena i sanitariia, (1)(1), 16-18 (1986-01-01)
Studies on Chrysanthemic Acid: Part VIII. Synthesis of Pyrethric Acids Part IX. Alternate Preparation of (+)-trans-pyrethric Acid and Methyl (+)-trans-2, 2-Dimethyl-3-(2'-carboxy-1' propenyl)-cyclopropanecarboxylate.
Matsui M, et al.
Agricultural and Biological Chemistry, 27(5), 373-380 (1963)
M Dondi et al.
Journal of chromatography. A, 859(2), 133-142 (1999-11-26)
The direct enantioseparation of chrysanthemic acid [2,2-dimethyl-3-(2-methylpropenyl)-cyclopropanecarboxylic acid] and its halogen-substituted analogues was systematically studied by HPLC using a terguride-based chiral stationary phase in combination with a UV diode array and chiroptical detectors. Isomers with (1R) configuration always eluted before
M Nishizawa et al.
Applied and environmental microbiology, 61(9), 3208-3215 (1995-09-01)
The gene coding for a novel esterase which stereoselectively hydrolyzes the (+)-trans (1R,3R) stereoisomer of ethyl chrysanthemate was cloned from Arthrobacter globiformis SC-6-98-28 and overexpressed in Escherichia coli. The cellular content of the active enzyme reached 33% of the total
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.