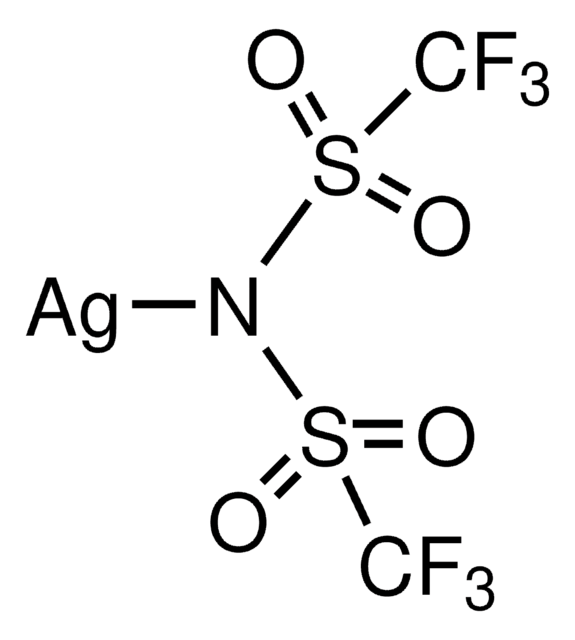
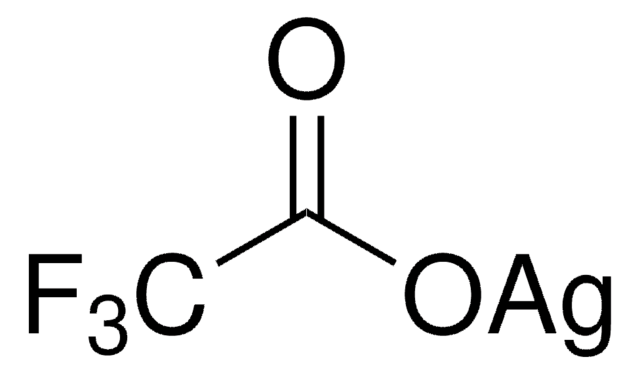
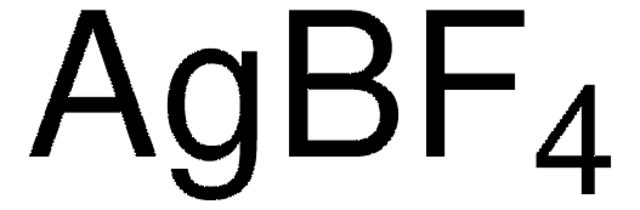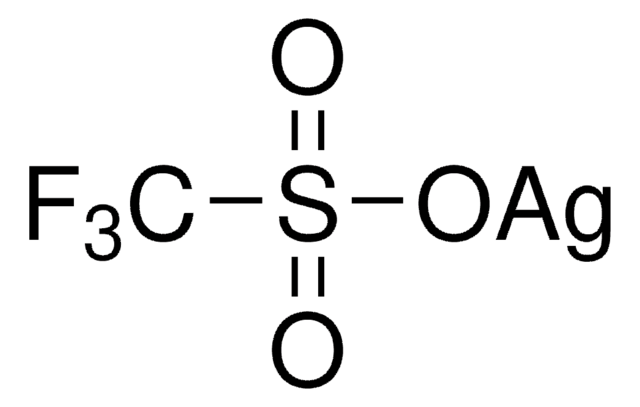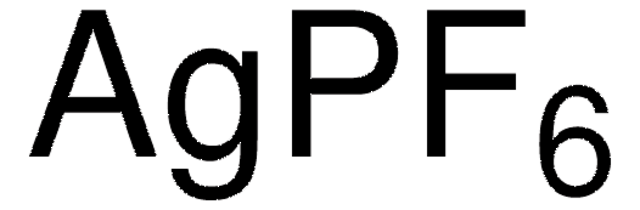추천 제품
Quality Level
분석
≥99%
형태
powder
반응 적합성
core: silver
reagent type: catalyst
mp
286 °C (lit.)
SMILES string
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
QRUBYZBWAOOHSV-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
It can also be used:
- To obtain olefins from secondary phosphates and thiophosphates.
- As a reagent in the etherification of alcohols with primary alkyl halides under mild conditions.
- To generate cationic rhodium catalysts from chlororhodium complexes for the hydrophosphination of acetylenes.
- As a catalyst for the preparation of silyl ethers by hydrosilylation of aldehydes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
문서
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently.
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.