추천 제품
Quality Level
분석
98%
형태
solid
불순물
≤2.0% water
mp
51-54 °C (lit.)
작용기
ketone
저장 온도
2-8°C
SMILES string
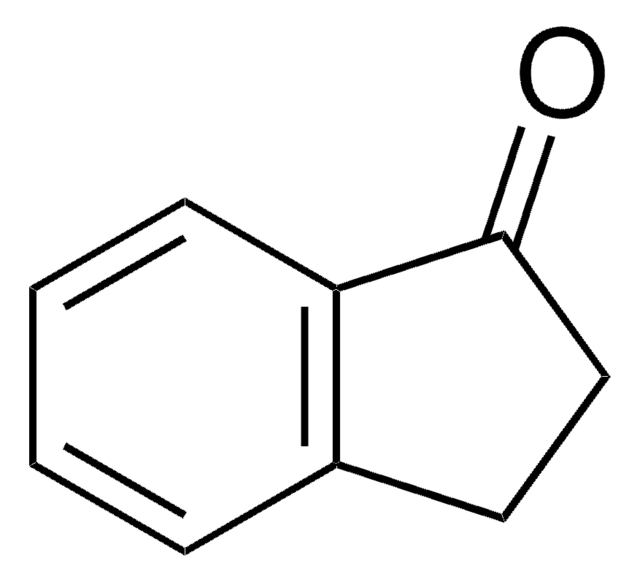
O=C1Cc2ccccc2C1
InChI
1S/C9H8O/c10-9-5-7-3-1-2-4-8(7)6-9/h1-4H,5-6H2
InChI key
UMJJFEIKYGFCAT-UHFFFAOYSA-N
유전자 정보
human ... CYP1A2(1544)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
2-Indanone undergoes TiCl4-Mg mediated coupling with CHBr3 to yield dibromomethyl carbinol. It reacts with 5,5-dimethyl-3-pyrazolidinone to yield 5,5-dimethyl-2-(1H-indenyl-2)-3-pyrazolidinone. 2-Indanone on photolysis by 266-nm one-photon excitation yields o-xylylene.
애플리케이션
2-Indanone was used as starting reagent in the synthesis of indene-fused porphyrins.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
212.0 °F - closed cup
Flash Point (°C)
100 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Spectroscopic studies on photochemical formation of o-xylylene in solution.
Fujiwara M, et al.
The Journal of Physical Chemistry A, 101(27), 4912-4915 (1997)
Tu-Hsin Yan et al.
Organic letters, 15(22), 5802-5805 (2013-11-07)
TiCl4-Mg can mediate addition of CHBr3 to a variety of aldehydes and ketones to form dibromomethyl carbinols and also be used to effect CBr3 transfer to carbonyl groups to form tribromomethyl carbinols. The successful application of TiCl4-Mg-promoted coupling of CHBr3
Timothy D Lash et al.
The Journal of organic chemistry, 76(13), 5335-5345 (2011-05-24)
Indene-fused porphyrins have been synthesized starting from 2-indanone. Knorr-type reaction of oximes derived from benzyl or tert-butyl acetoacetate with 2-indanone and zinc dust in propionic acid gave good yields of indenopyrroles. Treatment with N-chlorosuccinimide then gave 8-chloro derivatives, and these
Laura E Korhonen et al.
Journal of medicinal chemistry, 48(11), 3808-3815 (2005-05-27)
The purpose of this study was to determine the cytochrome P450 1A2 (CYP1A2) inhibition potencies of structurally diverse compounds to create a comprehensive three-dimensional quantitative structure-activity relationship (3D-QSAR) model of CYP1A2 inhibitors and to use this model to predict the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.