143065
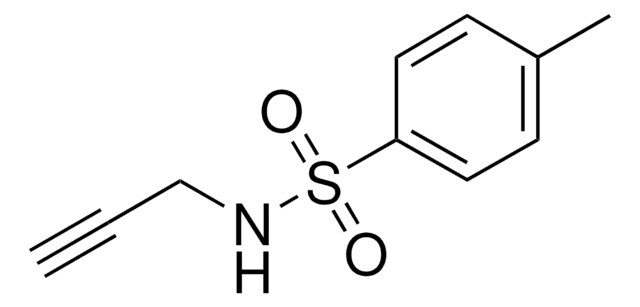
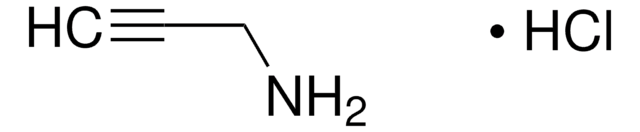
3-Dimethylamino-1-propyne
97%
동의어(들):
1-Dimethylamino-2-propyne, N,N-Dimethyl-2-propynylamine, N,N-Dimethylpropargylamine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
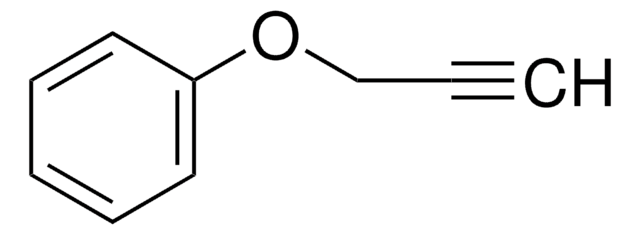
(CH3)2NCH2C≡CH
CAS Number:
Molecular Weight:
83.13
Beilstein:
1071222
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
일반 설명
3-Dimethylamino-1-propyne is a precursor for synthesis of mixed cuprate reagents.
애플리케이션
3-Dimethylamino-1-propyne was used in the synthesis of core shell systems based on noble metal nanoparticles coated by copolymers (polyacetylenes).
생화학적/생리학적 작용
3-Dimethylamino-1-propyne inactivates mitochondrial monoamine oxidase from bovine liver.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
17.6 °F - closed cup
Flash Point (°C)
-8 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
A L Maycock et al.
Biochemistry, 15(1), 114-125 (1976-01-13)
3-Dimethylamino-1-propyne irreversibly inactivates mitochondrial monoamine oxidase from bovine liver. The inactivation results in the loss of absorption in the 450-500-nm region of the flavine spectrum and a concomitant increase in absorbance at 410 nm. For the enzyme-bound adduct epsilon410 =
3-(Dimethylamino)-1-propyne: convenient precursor for a versatile mixed cuprate reagent.
Ledlie DB and Miller G.
The Journal of Organic Chemistry, 44(6), 1006-1007 (1979)
John R Hoffman et al.
ACS applied materials & interfaces, 12(17), 19944-19954 (2020-04-08)
Charged functional groups are often incorporated onto the surface of nanofiltration (NF) membranes to facilitate the selective rejection of multivalent ions over monovalent ions. However, since fouling-resistant surfaces tend to be electrically neutral, the incorporation of charged functionality exacerbates membrane
Ilaria Fratoddi et al.
Nanoscale research letters, 6(1), 98-98 (2011-06-30)
Noble metal nanoparticles of different sizes and shapes combined with conjugated functional polymers give rise to advanced core shell hybrids with interesting physical characteristics and potential applications in sensors or cancer therapy. In this paper, a versatile and facile synthesis
Francesca Nunzi et al.
The journal of physical chemistry. B, 110(15), 7682-7687 (2006-04-14)
The reaction of the bifunctional organic molecule 1-(dimethylamino)-2-propyne (DMAP) on the Si(100) surface has been investigated by density functional calculations employing a two-dimer cluster model. We found that, once in the physisorbed dative bonded well (-20.0 kcal mol(-1)), DMAP can
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.