추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
99%
refractive index
n20/D 1.533 (lit.)
bp
206-207 °C (lit.)
mp
15 °C (lit.)
solubility
alcohol: soluble
diethyl ether: soluble
fatty oils: soluble
water: slightly soluble
density
1.084 g/mL at 25 °C (lit.)
SMILES string
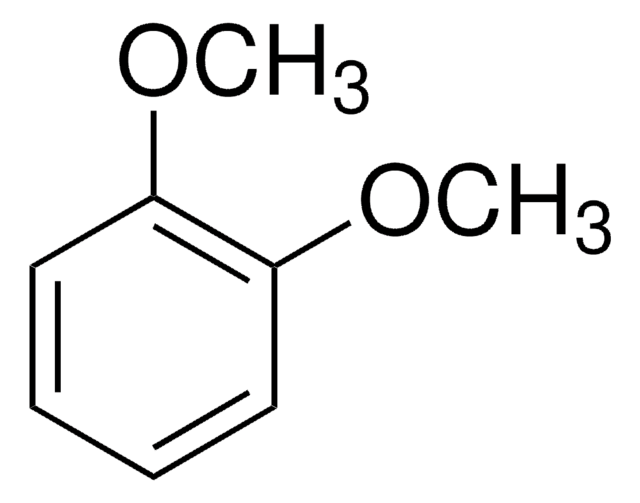
COc1ccccc1OC
InChI
1S/C8H10O2/c1-9-7-5-3-4-6-8(7)10-2/h3-6H,1-2H3
InChI key
ABDKAPXRBAPSQN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1,2-Dimethoxybenzene reacts with Li{N(SO2CF3)2} to yield molecular crystal [Li{N(SO2CF3)2}{C6H4(OCH3)2}2] having solid-state lithium ion conductivity. It is a potential pollinator attractant of the nocturnal moth Hadena bicruris.
애플리케이션
1,2-Dimethoxybenzene was used to investigate the electroantennogram response of vine weevil, Otiorhynchus sulcatus to plant volatiles.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Tariq A Akhtar et al.
Plant physiology, 162(1), 52-62 (2013-04-03)
White campion (Silene latifolia) is a dioecious plant that emits 1,2-dimethoxybenzene (veratrole), a potent pollinator attractant to the nocturnal moth Hadena bicruris. Little is known about veratrole biosynthesis, although methylation of 2-methoxyphenol (guaiacol), another volatile emitted from white campion flowers
Olfactory antennal responses of the vine weevil Otiorhynchus sulcatus to plant volatiles.
Tol RWHM and Visser JH.
Entomologia Experimentalis et Applicata, 102(1), 49-64 (2002)
Makoto Moriya et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(40), 13554-13560 (2013-08-14)
The molecular crystals [Li{N(SO2CF3)2}{C6H4(OCH3)2}2] and [Li{N(SO2CF3)2}{C6F2H2(OCH3)2}2] with solid-state lithium ion conductivity have been synthesized by the addition of two equivalents of 1,2-dimethoxybenzene or 1,2-difluoro-4,5-dimethoxybenzene to Li{N(SO2CF3)2}, respectively. Single-crystal X-ray diffraction analysis revealed the formation of ionic conduction paths with an
Karsten Seidelmann et al.
Journal of insect physiology, 49(12), 1125-1133 (2003-11-20)
Mature gregarious male desert locusts, Schistocerca gregaria, emit the courtship inhibition pheromone phenylacetonitrile. Wings and legs, in particular the fore wings, have been identified as the main releasing sites. Abdomen and head emit only trace amounts of this pheromone. In
Vincent J Chebny et al.
Organic letters, 11(11), 2253-2256 (2009-05-29)
Definitive X-ray crystallographic evidence is obtained for a single hole (or a polaron) to be uniformly distributed on the three equivalent 1,2-dimethoxybenzenoid (or veratrole) rings in the hexamethoxytriptycene cation radical. This conclusion is further supported by electrochemical analysis and by
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.