138622
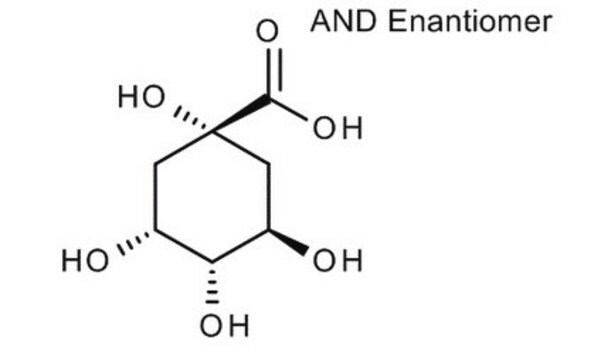
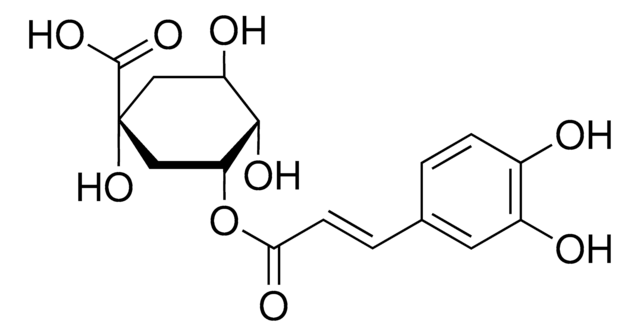
D-(−)-Quinic acid
98%
동의어(들):
(-)-Quinic acid, (1alpha,3R,4alpha,5R)-1,3,4,5-Tetrahydroxycyclohexanecarboxylic acid, D-(-)-Quinic acid
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C7H12O6
CAS Number:
Molecular Weight:
192.17
Beilstein:
2212412
EC Number:
MDL number:
UNSPSC 코드:
51113400
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
powder
광학 활성
[α]20/D −43.9°, c = 11.2 in H2O
작용기
carboxylic acid
hydroxyl
SMILES string
O[C@@H]1C[C@@](O)(C[C@@H](O)[C@H]1O)C(O)=O
InChI
1S/C7H12O6/c8-3-1-7(13,6(11)12)2-4(9)5(3)10/h3-5,8-10,13H,1-2H2,(H,11,12)/t3-,4-,5-,7+/m1/s1
InChI key
AAWZDTNXLSGCEK-WYWMIBKRSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
D-(-)-Quinic acid, a plant metabolite, is chiral building block used in multistep chemical synthesis of natural compounds.
애플리케이션
D-(−)-Quinic acid can be used as:
- A chiral selector electrolyte along with copper(II) sulfate. This electrolyte is utilized in chiral resolution DL-tartaric acid by ligand-exchange capillary electrophoresis method.
- A starting material in the synthesis of stereoisomers of 3,4,6-trihydroxyazepanes, 7-hydroxymethyl-3,4,5-trihydroxyazepanes, and 3,4,5-trihydroxyazepanes, as potential inhibitors of glycosidase.
- A precursor for the preparation of trihydroxy piperidine derivatives and (+)-proto-quercitol glycosidase inhibitors.
D-(-)-Quinic acid has been used as a standard to determine the composition of organic acids in bitter gentian teas and in developing cranberry fruit by HPLC. It may be used in the preparation of 3,4-O-isopropylidene-3(R),4(S)-dihydroxycyclohexanone.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Christopher J Potter et al.
Nature protocols, 6(8), 1105-1120 (2011-07-09)
In Drosophila, the GAL4/UAS/GAL80 repressible binary expression system is widely used to manipulate or mark tissues of interest. However, complex biological systems often require distinct transgenic manipulations of different cell populations. For this purpose, we recently developed the Q system
Tzenge-Lien Shih et al.
The Journal of organic chemistry, 72(11), 4258-4261 (2007-05-08)
Several new stereoisomers of 3,4,6-trihydroxyazepanes and 7-hydroxymethyl-3,4,5-trihydroxyazepanes as well as known 3,4,5-trihydroxyazepanes were synthesized as potent glycosidase inhibitors from D-(-)-quinic acid in an efficient manner. The key step employs dihydroxylation of protected chiral 1,4,5-cyclohex-2-enetriols under RuCl3/NaIO4/phosphate buffer (pH 7) condition
A unified asymmetric approach to substituted hexahydroazepine and 7-azabicyclo [2.2. 1] heptane ring systems from D (-)-quinic acid: Application to the formal synthesis of (-)-balanol and (-)-epibatidine
Albertini E, et al
Tetrahedron Letters, 38(4), 681-684 (1997)
d-(-)-Quinic acid: a chiron store for natural product synthesis
Barco A, et al
Tetrahedron Asymmetry, 8(21), 3515-3545 (1997)
Qiuling Li et al.
G3 (Bethesda, Md.), 6(10), 3351-3359 (2016-08-26)
Drosophila melanogaster is a powerful model organism for dissecting the molecular mechanisms that regulate sleep, and numerous studies in the fly have identified genes that impact sleep-wake cycles. Conditional genetic analysis is essential to distinguish the mechanisms by which these
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.