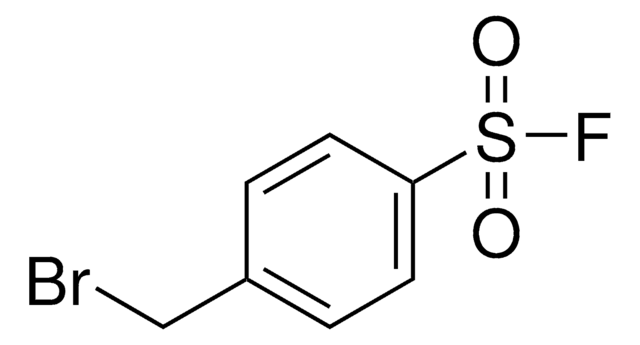
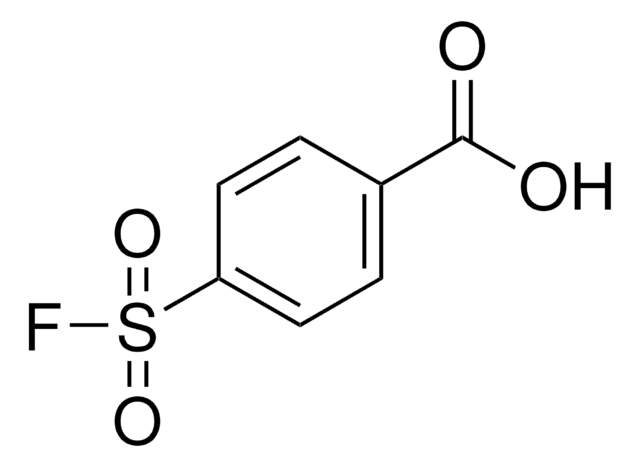
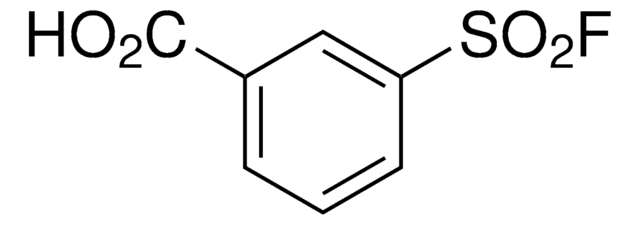
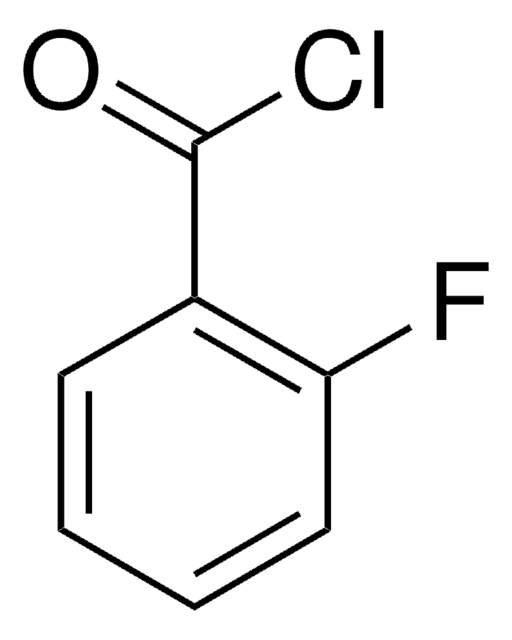
추천 제품
Grade
technical grade
Quality Level
분석
90%
반응 적합성
reaction type: click chemistry
bp
96-97 °C/0.7 mmHg (lit.)
mp
46-48 °C (lit.)
작용기
acyl chloride
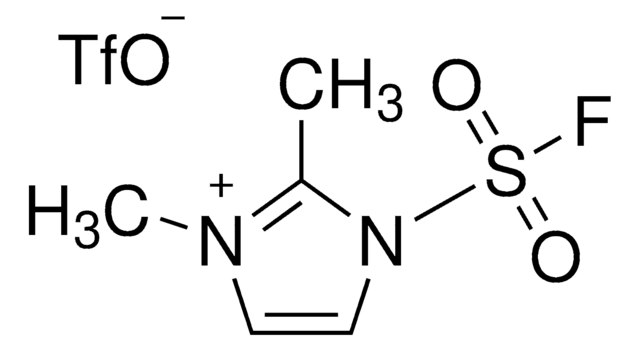
SMILES string
FS(=O)(=O)c1ccc(cc1)C(Cl)=O
InChI
1S/C7H4ClFO3S/c8-7(10)5-1-3-6(4-2-5)13(9,11)12/h1-4H
InChI key
JMTAYFNTRRLWQG-UHFFFAOYSA-N
애플리케이션
4-(Fluorosulfonyl)benzoyl chloride was used as reagent in the synthesis of irreversible adenosine A1 antagonist 8-cyclopentyl-3-N-[3-((3-(4-fluorosulphonyl)benzoyl)-oxy)-propyl]-1-N-propyl-xanthine.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
J E van Muijlwijk-Koezen et al.
Bioorganic & medicinal chemistry letters, 11(6), 815-818 (2001-03-30)
A new preparative synthetic route for the irreversible adenosine A1 antagonist 8-cyclopentyl-3-N-[3-((3-(4-fluorosulphonyl)benzoyl)-oxy)-propyl]-1-N-propyl-xanthine (FSCPX, 1) is described. The availability of ample amounts of the irreversible antagonist FSCPX allowed us to use FSCPX as a research tool for adenosine A1 receptors in
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.