10905
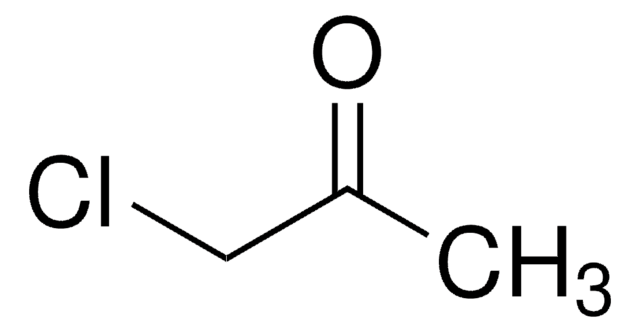
Chloroacetone
produced by Wacker Chemie AG, Burghausen, Germany, ≥96.0% (GC)
동의어(들):
MCA, 1-Chloropropan-2-one, Chloro-2-propanone
About This Item
추천 제품
Grade
produced by Wacker Chemie AG, Burghausen, Germany
Quality Level
분석
≥96.0% (GC)
포함
~0.1% Drapex 39 as stabilizer
refractive index
n20/D 1.432 (lit.)
bp
120 °C (lit.)
solubility
H2O: soluble 10 parts
alcohol: miscible
chloroform: miscible
diethyl ether: miscible
density
1.162 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
CC(=O)CCl
InChI
1S/C3H5ClO/c1-3(5)2-4/h2H2,1H3
InChI key
BULLHNJGPPOUOX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
기타 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
95.0 °F - closed cup
Flash Point (°C)
35 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.