애플리케이션
Collagenase from
Clostridium histolyticum has been used:
- in the preparation of collagenase solution for the digestion of tumor tissues
- as a supplement in phosphate-buffered saline for the digestion of extirpated mesenteric fat tissue
- as a supplement in Roswell Park Memorial Institute-1640 medium (RPMI-1640) for the digestion of colon segments
- for the digestion of endometrium tissues
- in the preparation of enzyme A and enzyme B for the digestion of decapsulated testes
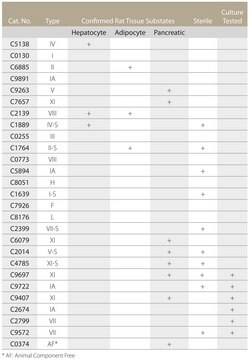
Collagenase has been used in the preparation of arterial tissue for the study of Advanced Glycosylation End Products (AGE). The enzyme has also been used along with other proteases for the disaggregation of human tumor, mouse kidney, human brain, lung epithelium and many other tissues. It is also effective in liver and kidney perfusion studies, digestion of pancreas, and isolation of nonparenchymal hepatocytes. This enzyme is tested for suitability for the release of hepatocytes, at approx. 1 mg/mL conc., in a total volume of 100 mL for each rat liver.
Suitable for use in preparation of single cell suspension for sequencing.
생화학적/생리학적 작용
Clostridium histolyticum produces two classes of collagenases. Clostridial collagenases are more efficient than mammalian collagenases. It cleaves at multiple cleavage sites within the collagen triple helix. Clostridial collagenases is implicated in the bacterial invasion in gas gangrene.
Collagenase is activated by four gram atom calcium per mole enzyme. It is inhibited by ethylene glycol-bis(beta-aminoethyl ether) - N, N, N′,N′-tetraacetic acid, beta-mercaptoethanol, glutathione, thioglycolic acid and 8-hydroxyquinoline.
Effective release of cells from tissue requires the action of collagenase enzymes and the neutral protease. Collagenase is activated by four gram atom calcium (Ca2+) per mole enzyme. The culture filtrate is thought to contain at least 7 different proteases ranging in molecular weight from 68-130 kDa. The pH optimum is 6.3-8.8. The enzyme is typically used to digest the connective components in tissue samples to liberate individual cells. Collagenase treatment can cause some cells to die. Typically, concentrations varying from 0.1 to 5 mg/mL are used for digestion. The duration of reaction varies from 15 minutes to several hours and yields satisfactory cell dissociation without causing too much cell death. Krebs Ringer buffer with calcium and BSA is preferred and Zn2+ is required for activity.
주의사항
As supplied, this product is stable for 6 years at -20°C. There is no loss in FALGPA or protease activity in 30 days at 37°C, 50°C and -20°C. Solutions of crude collagenase are stable if frozen quickly in aliquots (at 10 mg/mL) and kept frozen at -20°C. Further freeze-thaw cycles will damage the solution. The product retains 100% activity over 7 hours when held on ice.
단위 정의
One collagen digestion unit (CDU) liberates peptides from collagen from bovine achilles tendon equivalent in ninhydrin color to 1.0 μmole of leucine in 5 hours at pH 7.4 at 37 °C in the presence of calcium ions. One FALGPA hydrolysis unit hydrolyzes 1.0 μmole of furylacryloyl-Leu-Gly-Pro-Ala per min at 25°C. One Neutral Protease unit hydrolyzes casein to produce color equivalent to 1.0 μmole of tyrosine per 5 hr at pH 7.5 at 37°C. One Clostripain Unit hydrolyzes 1.0 μmole of BAEE per min at pH 7.6 at 25°C in the presence of DTT.
제조 메모
Solutions are typically prepared at 1-2 mg/mL in TESCA buffer (containing 50 mM TES, 0.36 mM Calcium chloride, pH 7.4 at 37°C.
This product also contains clostripain, neutral protease and tryptic activities.