C9268
Carboxypeptidase A from bovine pancreas
(Type II-PMSF treated), ≥50 units/mg protein, ready-to-use solution
Synonym(s):
Carboxypolypeptidase, Peptidyl-L-amino-acid hydrolase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
grade
Proteomics Grade
Quality Level
form
ready-to-use solution
quality
(Type II-PMSF treated)
specific activity
≥50 units/mg protein
mol wt
~35 kDa
purified by
2× crystallization
impurities
≤0.05 BTEE units/mg protein chymotrypsin
≤10 BAEE units/mg protein trypsin
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Carboxypeptidase A from bovine pancreas has been used in a study to investigate the expression of a soluble and activatable form of bovine procarboxypeptidase A in Escherichia coli. Carboxypeptidase A from bovine pancreas has also been used in a study to investigate the isolation and partial characterization of precursor forms of ostrich carboxypeptidase.
The enzyme from Sigma has been used as a comparison to study the specificity of Metarhizium anisopliae carboxypeptidase A (MeCPA). MeCPA had been genetically engineered to facilitate the removal of polyhistidine tags from the C-termini of recombinant proteins. It has also been used to de-tyrosinate α-tubulin, in vitro, in order to induce high affinity to ethyl-N-phenylcarbamate (EPC) sepharose.
Biochem/physiol Actions
Carboxypeptidase as isolated from bovine pancreas glands is a metalloenzyme that contains 1 g atom of zinc per mole of protein. It catalyzes the hydrolysis of the carboxyl-terminal peptide bond in peptides and proteins. It is primarily specific to aromatic and hydrophobic side chains such as phenylalanine, tryptophan or leucine. The enzyme also exhibits esterase activity. It is inhibited by beta-phenylpropionate and indole acetate.
Unit Definition
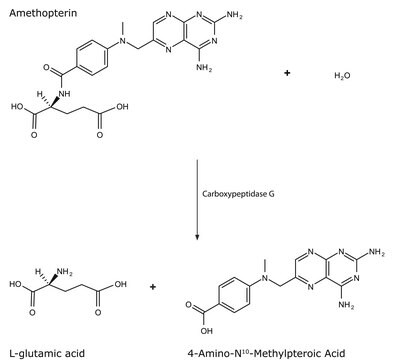
One unit will hydrolyze 1.0 μmole of hippuryl-L-phenylalanine per min at pH 7.5 at 25 °C.
Preparation Note
Treated with phenylmethylsulfonyl fluoride to eliminate trypsin and chymotrypsin activity. Dialyzed and recrystallized: aqueous suspension with toluene added.
Analysis Note
Protein determined by E1%/278
inhibitor
Product No.
Description
Pricing
substrate
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Brian P Austin et al.
Protein expression and purification, 77(1), 53-61 (2010-11-16)
Carboxypeptidases may serve as tools for removal of C-terminal affinity tags. In the present study, we describe the expression and purification of an A-type carboxypeptidase from the fungal pathogen Metarhizium anisopliae (MeCPA) that has been genetically engineered to facilitate the
Bodo Wiesler et al.
The Plant journal : for cell and molecular biology, 32(6), 1023-1032 (2002-12-21)
Auxin controls the orientation of cortical microtubules in maize coleoptile segments. We used tyrosinylated alpha-tubulin as a marker to assess auxin-dependent changes in microtubule turnover. Auxin-induced tyrosinylated alpha-tubulin, correlated with an elevated sensitivity of growth to antimicrotubular compounds such as
Kaia Kukk et al.
Journal of biotechnology, 231, 224-231 (2016-06-19)
Vertebrate prostaglandin H synthases (PGHSs) are membrane-bound disulphide-containing hemoglycoproteins. Therefore, eukaryotic expression systems are required for the production of recombinant PGHSs. Recently we announced the expression of human PGHS-2 (hPGHS-2) in the yeast Pichia pastoris. Here we report improved production
Sahar I Da'as et al.
Blood, 119(15), 3585-3594 (2012-03-01)
We used the opportunities afforded by the zebrafish to determine upstream pathways regulating mast cell development in vivo and identify their cellular origin. Colocalization studies demonstrated zebrafish notch receptor expression in cells expressing carboxypeptidase A5 (cpa5), a zebrafish mast cell-specific
Anne-Lise Marie et al.
Analytica chimica acta, 800, 103-110 (2013-10-15)
The present study describes a reproducible and quantitative capillary zone electrophoresis (CZE) method, which leads to the separation of nine forms (native, oxidized and glycated) of human serum albumin (HSA). In an attempt to identify the different species separated by
Protocols
Enzymatic Assay of Carboxypeptidase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service