906107
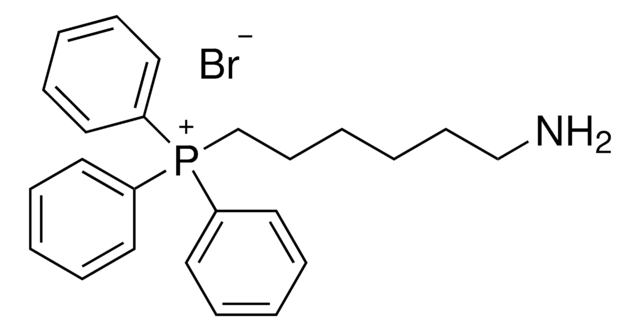
(9-Aminononyl)triphenylphosphonium bromide
90%
Synonym(s):
Aminononane TPP mitochondrial tag, Mitochondria-targeting probe building block, Phosphonium lipocation tag
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H35BrNP
Molecular Weight:
484.45
UNSPSC Code:
12352200
Recommended Products
Assay
90%
form
powder
storage temp.
2-8°C
SMILES string
NCCCCCCCCC[P+](C1=CC=CC=C1)(C2=CC=CC=C2)C3=CC=CC=C3.[Br-]
Application
Mitochondria perform several functions in the cell, and dysfunction has been implicated in various organ systems and disease states, including neurodegenerative disorders, cardiovascular diseases, and cancers. The targeting of small molecules to the mitochondria can help to probe and better understand this relationship or to increase therapeutic efficacy by concentrating a drug at its site of action and reducing off-target effects. This bromohexane triphenylphosphine (TPP) tag is conjugated to small molecules to localize them to the mitochondria in cells where the TPP cations facilitate both cellular uptake and accumulation in the mitochondria.
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Marcel Culcasi et al.
Journal of medicinal chemistry, 56(6), 2487-2499 (2013-02-27)
A series of mitochondria targeted α-aminophosphonates combining a diethoxyphosphoryl group and an alkyl chain-connected triphenylphosphonium bromide tail were designed and synthesized, and their pH-sensitive (31)P NMR properties and biological activities in vitro and in vivo were evaluated. The results showed
Changwook Lee et al.
Journal of the American Chemical Society, 137(13), 4358-4367 (2015-03-19)
The mitochondrial pool of Hsp90 and its mitochondrial paralogue, TRAP1, suppresses cell death and reprograms energy metabolism in cancer cells; therefore, Hsp90 and TRAP1 have been suggested as target proteins for anticancer drug development. Here, we report that the actual
Wen Zhang et al.
Analytical chemistry, 89(12), 6840-6845 (2017-05-16)
Mitochondrial morphology regulated by fusion and fission processes determines mitochondrial function and cell fate. Some studies showed hyperfused mitochondria could induce apoptosis in cancer cells, but the relevant molecular mechanisms remain elusive. Superoxide (O2•-) and pH play vital roles in
Friedrich Kreyenschmidt et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 24(5), 1168-1177 (2017-11-08)
The combination of CoCl2 and 1,3-dienes is known to catalyze challenging alkyl-alkyl cross-coupling reactions between Grignard reagents and alkyl halides, but the mechanism of these valuable transformations remains speculative. Herein, electrospray-ionization mass spectrometry is used to identify and characterize the
Yon Ju-Nam et al.
Organic & biomolecular chemistry, 4(23), 4345-4351 (2006-11-15)
We report the synthesis and structural characterisation of a new family of stable phosphonioalkylthiosulfate zwitterions, R3P+ (CH2)nS2O3- (R = Ph or Bu, n = 3,4,6, 8 or 10) which behave as cationic masked thiolate ligands with applications in the functionalisation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service