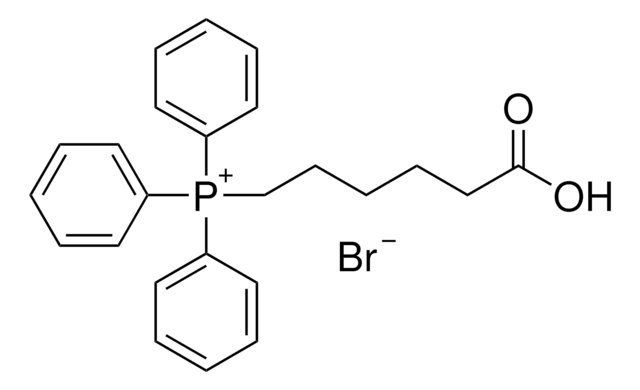
349720
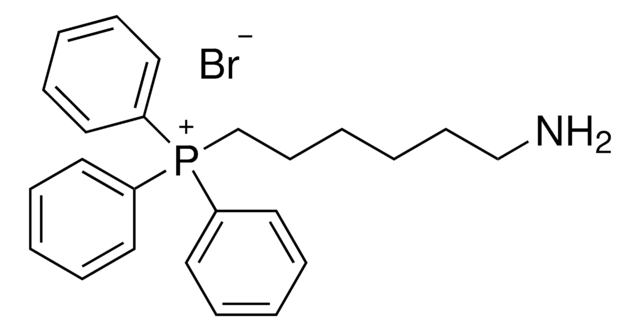
(3-Carboxypropyl)triphenylphosphonium bromide
98%
Synonym(s):
(4-Hydroxy-4-oxobutyl)triphenylphosphonium bromide
About This Item
Recommended Products
Quality Level
Assay
98%
reaction suitability
reaction type: C-C Bond Formation
mp
244-247 °C (lit.)
functional group
carboxylic acid
phosphine
SMILES string
[Br-].OC(=O)CCC[P+](c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C22H21O2P.BrH/c23-22(24)17-10-18-25(19-11-4-1-5-12-19,20-13-6-2-7-14-20)21-15-8-3-9-16-21;/h1-9,11-16H,10,17-18H2;1H
InChI key
NKVJKVMGJABKHV-UHFFFAOYSA-N
Application
- Piperamide analogs as histone deacetylase (HDAC) inhibitors with antitumor activity
- Boron-containing benzoxaboroles as antimalarial agents
- Methyl alkenyl quinolones as antimycobacterial agents
- Solandelactone E via Sharpless epoxidation, Taber cyclopropanation, chemoselective reductions and lithiation-borylation-allylation sequence
- Organotin compounds as antifungal agents
- Oleanolic acid derivatives as inhibitors of protein tyrosine phosphatase 1B for negative regulation of insulin pathway (a promising target for treatment of diabetes and obesity)
- 11-oxa prostaglandin analogs with ocular hypotensive activity
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[3-(Ethoxycarbonyl)propyl]triphenylphosphonium bromide 97%](/deepweb/assets/sigmaaldrich/product/structures/101/490/c9797f74-da99-4610-8812-8572dc35c41d/640/c9797f74-da99-4610-8812-8572dc35c41d.png)