Autophagy Mitophagy in Tumor Cell Survival Death
Chandra Mohan, Ph.D.
MilliporeSigma
Autophagy is a highly regulated process that is involved in cell growth, development, and death. In autophagy, cells destroy their own cytoplasmic components in a very systematic manner and recycle them. In mammalian cells, autophagy occurs constitutively at basal rates, and its primary function is to protect cells under stress conditions. Under conditions of starvation, cells use this process to reallocate nutrients from less important to more essential processes required for survival. By degrading cytoplasmic components autophagy releases amino acids for protein synthesis and fatty acids for oxidation in mitochondria to produce energy for survival. However, if cellular damage becomes irreparable, cells can destroy themselves completely by autophagy. mTOR is a key regulator of autophagy. Under normal physiological conditions, mTOR inhibits autophagy, but, under extreme nutritional deficiency mTOR can be inactivated, which leads to autophagy activation. Several processes can be classified under the general term “autophagy”. However, one common element of autophagy pathways involves the importation of cytoplasmic components into the lysosome.
Three Types of Autophagy
Based on the pathway via which cargo marked for degradation is delivered to the lysosome, three types of autophagy have been described (Figure 1). They are chaperone-mediated autophagy, microautophagy, and macroautophagy. Chaperone-mediated autophagy (CMA) involves the direct translocation of cytosolic proteins across the lysosomal membrane. CMA mediates the degradation of a selective subset of cytosolic proteins in lysosomes. For a protein to be degraded via CMA KFERQ motif is required, which is recognized by cytosolic chaperone (HSC70). In addition, several modulatory chaperones (Bag1, Hip, Hop, and HSP40) act in concert to bring substrate proteins to lysosomal surface. The substrate protein–chaperone complex docks at the lysosomal membrane with the help of LAMP-2A. Substrate proteins are unfolded prior to their internalization, which is not required in other forms of autophagy.
The second autophagy process is microautophagy. During microautophagy, cytoplasm is sequestered directly at the lysosomal surface by separation and/or invagination of the lysosomal membrane. The invaginated vacuole internalizes cytoplasmic components for subsequent degradation. Microautophagy also involves the direct engulfment of organelles, such as the peroxisome and the nucleus.
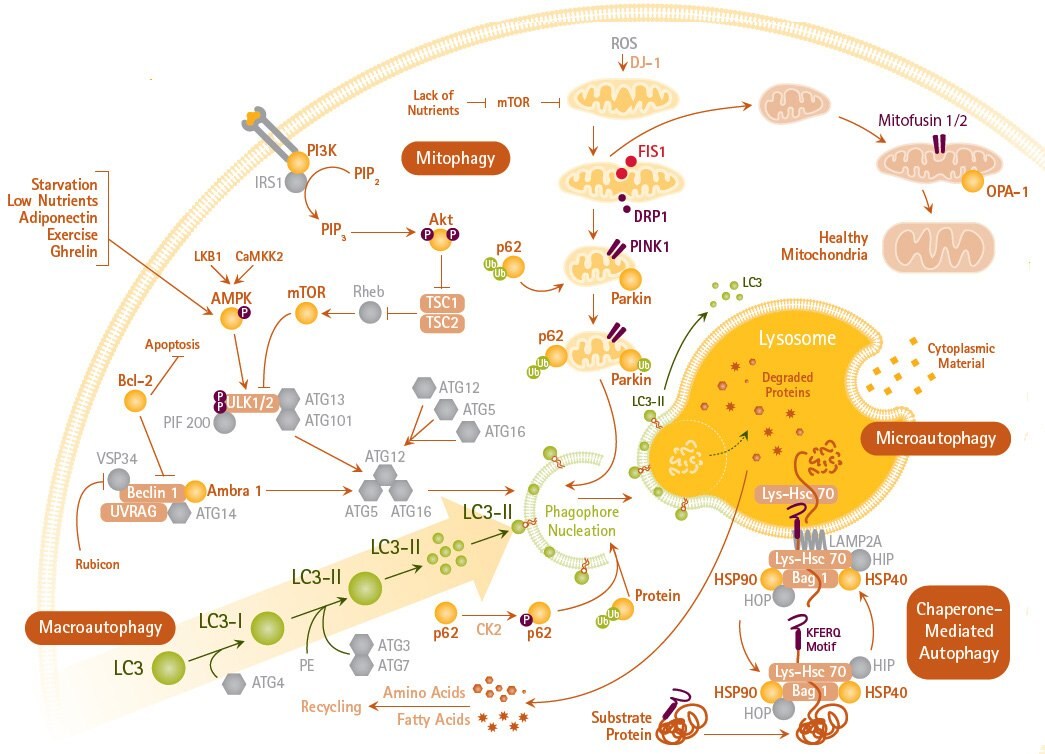
Figure 1.Pathways of Macro-, Micro-, and Chaperone-mediated Autophagy and Mitophagy
The third type of autophagy is macroautophagy, which it is the major autophagy pathway in mammalian cells. Macroautophagy is an evolutionarily conserved process by which cells maintain homeostasis by degrading entire organelles and long-lived proteins. In here, the sequestering of membrane is distinct from lysosome and it involves the formation of autophagosome that fuses with lysosome, which provides the hydrolytic enzyme machinery. The fused structure is called autophagolysosome. The maturation of autophagolysosome requires acidification by H1-ATPase. Hence, several inhibitors of H1-ATPase, such as Bafilomycin A1 are shown to diminish autophagy.
Steps Involved in Macroautophagy
The process of autophagy can be divided into several phases. In the initial phase, the cell senses signals released in response to lack of nutrients, hypoxia, or other forms of stress. These signals induce macroautophagy, either via inhibition of mTOR or by AMPK activation of the ULK1/2 kinase complex. mTOR activity negatively regulates autophagy, which may involve AMP kinase induced phosphorylation of mTOR on specific sites. Autophagy can also be induced in mTOR-independent manner by lowering the levels of myo-inositol-1, 4, 5-triphosphate in cells by using lithium, carbamezapine, or sodium valproate.
The second phase involves the formation of the phagophore, a subcellular structure. The origin of the phagophore membrane is unclear, but a contribution of the endoplasmic reticulum, Golgi, mitochondria, and endosomes has been suggested. The ULK1,2/Atg13 complex, by yet to be known mechanism, catalyzes the scaffolding of Atg protein complexes onto the phagophore membrane. The Atg14L/Vps34 protein complex generates phosphatidylinositol triphosphate, which recruits still more Atg complexes to the membrane.
During the third phase, elongation of the phagophore to form the autophagosome occurs, which is mediated by two protein conjugation systems that are reminiscent of the ubiquitin conjugation pathway. Atg7 is an E1-like activating enzyme, which along with Atg10 (E2-like conjugating enzyme), links Atg12 to Atg5 and Atg16L. This latter complex has an E3-like ligase activity that conjugates phosphatidylethanolamine (PE) to microtubule-associated protein 1A/1B-light chain 3 (LC3) to form LC3-PE conjugate (known as LC3-II). LC3-II is recruited to autophagosomal membranes and is translocated to autolysosomal lumen where it is degraded. The turnover of LC3-II mirrors starvation-induced autophagic activity, and the detection of LC3, either by immunofluorescence or by immunoblotting is considered as a reliable method for monitoring autophagy.
Mitophagy
Another important part of autophagy process is the selective degradation of mitochondria, known as mitophagy, which involves the participation of about 40 different genes. Although mitochondria can be engulfed along with cytosolic contents during “bulk autophagy,” cells can also selectively degrade damaged or superfluous mitochondria by mitophagy. Hence, mitophagy can be viewed as an important quality control process to eliminate damaged mitochondria or those with mutations in their DNA. Mitophagy also helps to regulate the number of mitochondria in response to diminished metabolic demands under different physiological conditions.
In order to undergo mitophagy, mitochondria must be separated from the mitochondrial network and damaged mitochondria are not allowed to fuse with mitochondrial reticulum. Mitochondria can also undergo fission and fusion based on local physiological demands. In mammalian cells, mitochondrial fission is achieved with the help of dynamin-related protein 1 (Drp1) that forms a multimeric complex to wrap outer mitochondrial membrane and forces membrane split. For mitochondrial fusion to occur Mitofusin 1 and 2 and optic atrophy 1 (Opa1) are required. These proteins help to fuse outer and inner mitochondrial membranes, respectively. Mitochondrial fission and fusion help to distribute mitochondrial DNA and proteins throughout the mitochondrial network.
In mammalian cells, mitophagy is achieved by a close interaction of PTEN-induced putative kinase 1 (PINK1), a mitochondrial Serine/Threonine kinase and Pakin, an E3 ubiquitin ligase. Both PINK1 and Parkin are shown to accumulate in damaged mitochondria. PINK1 facilitates binding of Parkin to depolarized mitochondria to selectively induce mitophagy. Uncouplers of oxidative phosphorylation, such as carbonyl cyanide m-chlorophenylhydrazone, are shown to induce mitophagy. Depolarization can reduce the level of both Opa1 and Mitofusins, which allows mitochondrial fragmentation to proceed. Depolarization also causes PINK1 and Parkin to associate with Miro, a mitochondrial Rho-GTPase. PINK1 phosphorylates Miro that allows it to undergo proteasomal degradation. Loss of Miro releases kinesin from mitochondria resulting in reduced mitochondrial motility.
Interplay Between Autophagy and Apoptosis in Tumor cells
In contrast to normal cells, tumors cells usually exist in a hypoxic environment that is low in nutrients. On one hand, autophagy may serve as a tumor suppressor mechanism whereby tumor cells progressively “eat” themselves under these unfavorable metabolic conditions. On the other hand, one of their most remarkable achievements is to activate autophagy in response to hypoxic stress, which enables their long-term survival, particularly when apoptosis is defective. With mutated Bcl-2, tumor cells can conveniently survive chemotherapy by employing protective autophagic mechanisms. Tumor cells can progressively eat themselves under prolonged stress, becoming less than one-third their normal size, but these “dormant” tumor cells retain the capacity to return to their normal size and resume cell proliferation when normal growth conditions are restored. Thus, both activation and inhibition of autophagy may hold promise for improved cancer chemotherapy.
Correlation between autophagy and apoptosis is an emerging topic of interest, especially in the field of tumor biology. On one hand, autophagy induces cell death by degrading essential components, but on the other it can facilitate survival of cancer cells under unfavorable metabolic conditions. Various stimuli that can induce apoptosis can also trigger autophagy. Here autophagy may precede apoptosis and may result from lower degree of stress. When apoptosis is suppressed it can lead to exacerbated autophagy. Hence, autophagy can be considered as a strategy by which cells cope up with stress, but if apoptosis is commenced then autophagy can be inactivated. The mitochondrion has been noted as a switch between apoptosis and autophagy. When autophagy is blocked and the damaged mitochondria are not eliminated, they suffer a loss of transmembrane potential (ΔΨm), which can lead to apoptosis. Mitochondria are considered as the ‘battleground’ where survival and death signals converge to determine whether or not cells undergo apoptosis. Marino et al (2014) suggest that mitochondria are particularly prone to apoptosis and their removal by autophagy can increase the threshold for apoptosis induction.
A reduction in ΔΨm has been linked to increased ubiquitination of voltage-dependent anion channel (VDAC) and Mitofusin 1 and 2, which leads to a reduction in mitophagy. It is also known that mitochondrial fragmentation via fission is essential for mitophagy, which prevents unwarranted removal of functional, healthy mitochondria. Mitochondria from starved cells underdo dissipation in their ΔΨm, but do not undergo fragmentation and mitophagy.
Under conditions of extreme stress when cells fail to perform autophagy, caspases are activated and apoptosis is allowed to proceed. Activated caspases digest and eliminate several autophagy-related proteins, such as Atg3, Beclin- 1, and AMBRA1, which can lead to accelerated apoptosis. In addition, as a result of caspase-induced fragmentation of autophagy proteins, selected fragments can acquire pro-apoptotic functions. For example, the cleavage of Beclin-1 can produce a carboxy-terminal fragment that can localize to mitochondria and causes the release of cytochrome c.
Autophagy Pathways as Therapeutic Targets
Autophagy can be pharmacologically induced by inhibiting negative regulators such as TOR with rapamycin or blocking the activity of anti-apoptotic protein Bcl-2 with inhibitors, and blocking the activity of IP3 receptor with xestospongin B. Autophagy can also be inhibited by 3-methyladenine that targets the class III PI3K involved in autophagosome formation; or by targeting the fusion of autophagosomes with lysosomes with bafilomycin A1 or chloroquine. A better understanding of autophagy will allow us to develop therapeutic agents to either increase or decrease the extent of this process. A number of disease states, including those where mutant proteins cause pathological changes, could become target of autophagy inducing agents. Some of the examples include Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease where undesirable aggregates of proteins are causative factors of disease. Armed with newly developed immunoreagents, small molecule inhibitors and autophagy assays, researchers in diverse fields are poised to shed light on the potential effects of perturbing autophagy pathways on the progression of these diseases.
REFERENCES
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?