Review of O-Linked Glycoproteins
O-Linked glycoproteins are usually large proteins with a molecular mass of >200 kDa. Glycosylation generally occurs in high-density clusters and may represent as much as 50-80% of the overall mass.
Structures
O-Linked glycans are most commonly attached to the peptide chain through serine (Ser) or threonine (Thr) residues. While O-linkage does link primarily to peptide residues through a hydroxyl group, there is no consensus sequence required. Tyrosine (Tyr), hydroxylysine (Hydroxy-Lys), or hydroxyproline (Hydroxy-Pro) may also be the peptide site of O-linked glycosylation.
The most common O-linked glycans are the mucin-type glycans, which contain an initial GalNAc residue. There are eight mucin-type core structures (Figure 1). Even with common mucin-type cores, O-linked glycans tend to be very heterogeneous, and there are other structures possible, in addition to several sialylated core structures. However, O-linked glycans are commonly linear or biantennary and have comparatively less branching than N-glycans.

Figure 1.Core Structures of mucin-type O-glycans.
Mucins are glycoproteins that contain large numbers of high-density clusters of O-linked glycans. The mucin-type glycans of these proteins frequently form cross-linked connections in aqueous solutions, resulting in a high viscosity gel (mucus). Mucins can be secreted, but may also be membrane bound and form glycan-dense areas on the cell surface.
In addition to mucin-type glycans, O-linked glycans may incorporate sugars other than GalNAc as the initial sugar bound to the serine/threonine residues. Examples of alternative O-linked glycans are:
- Nuclear and cytoplasmic glycoproteins that contain GlcNAc as the initiating sugar.
- Fibrinolytic and coagulation factors that contain fucose as the initiating sugar.
- Mannoproteins that are typical to yeasts and that incorporate mannose as the initiating sugar. O-Mannosyl glycans are also found in human α-dystroglycan and other nervous system glycoproteins.
- Glycosaminoglycans (GAGs) that are components of proteoglycan structures and contain xylose bound exclusively to serine residues.
- Plant cell wall extensins that contain both arabinose attached to hydroxyproline and galactose attached to serine.
- Plant arabinogalactans that are attached to the hydroxyproline within the peptide backbone through O-linked galactose or glucose.
- Galactose and αGlc(1→2)Gal residues that are bound to hydroxylysine within the triple helix structures of collagen. Complement factor C1q also contains αGlc(1→2)Gal-hydroxyproline sequences.
- Glycogenin, a protein precursor required for glycogen synthesis, contains glucose O-linked to tyrosine; the initial glucose is subsequently elongated by glycogen synthase to generate glycogen.
Biosynthesis and Degradation
O-linked glycosylation is a true post-translational event that occurs in the golgi, and no oligosaccharide precursor is required for protein transfer. The serine/threonine residues are modified directly by covalent addition of N-acetylgalactosamine residues. Initiation of mucin-type O-glycosylation is dependent upon polypeptide N-acetylgalactosyl transferase (ppGalNAcT); at least twelve mammalian ppGalNAcT isozymes have been identified. The first step in the synthesis of mucin-type glycans requires catalysis by a ppGalNAcT in the presence of UDP-GalNAc as the carbohydrate donor. Subsequent elongation and termination of O-linked glycans is carried out by several glycosyltransferases. The relative expression and subcellular distribution of the various glycosyltransferases determine the outcome of O-glycan biosynthesis. Termination of O-linked glycans usually includes Gal, GlcNAc, GalNAc, Fuc, or sialic acid. By far the most common modification of the core Gal-β(1→3)- GalNAc is mono-, di-, or trisialylation (Core 1 and 2) (Figure 2). A less common, but widely distributed O-linked hexasaccharide structure contains β(1→4)-linked Gal and β(1→6)-linked GlcNAc, as well as sialic acid.

Figure 2.Disialylated (top) and trisialylated (bottom) O-linked Core 1 glycans.
O-glycan degradation requires α-N-acetylgalactosaminidase in addition to the same exoglycosidases needed for N-glycan degradation.
Functions
Secreted mucins at the apical membrane of epithelial cells can link through disulfide bonds and capture water molecules, forming a mucus membrane. The membrane physically protects the cell from hostile environmental factors such as stomach acids and circulating proteases. Mucin secretion by salivary glands provides lubrication for swallowing. Mucins also block infection by pathogens by presenting a wall of O-linked glycans to attract and bind bacterial carbohydrate binding receptors. Many bacterial pathogens express adhesins, carbohydrate-recognition proteins specific for cell surface O-glycan structures that function as receptors for binding and infecting host cells. The adhesins bind to the mucin surface glycans, preventing further progress by the pathogen; the bound pathogen can then be eliminated.
Glycosylation by a single GlcNAc moiety is a unique form of O-glycosylation, in that it has been shown to be dynamic, rather than static like other types of O-linked glycosylation. This modification is reversible and catalyzed by the enzymes uridine diphospho-N-acetylglucosmine: polypeptide β-N-acetylglucosaminyltransferase (O-GlcNAc transferase) and neutral β-N-acetylglucosaminidase (O-GlcNAcase). Proteins that are O-linked with GlcNAc may alternatively be phosphorylated at the same peptide site, and similarly to phosphorylation, O-GlcNAc processing has been associated with cellular signaling events, including insulin signaling and RNA transcription regulation.1,2
More complex O-glycans serve other functions. ZP glycoproteins are O-linked glycans present in high concentrations in the zona pellucida surrounding mammalian eggs. Human ZP matrix contains four ZP glycoproteins, while the mouse ZP matrix has only three.3 The roles of ZP glycoproteins have not been fully determined but are thought to be associated with sperm reception.
O-Linked glycans are also involved in hematopoiesis and inflammation response mechanisms. The P-selectin glycoprotein ligand 1 (PSGL-1) contains, among other glycans, a Core 2 O-glycan capped with sialyl Lewis X (C2‑O-sLeX, Figure 3). PSGL-1 is the primary adhesion target for P-selectin and a target for E-selectin, which are involved in leukocyte rolling and recruitment into sites of inflammation.4

Figure 3.Structure of the O-linked Core 2 glycan with attached sialyl Lewis X (sLex) moiety.
The human ABO blood antigens are small O-linked glycans that may be attached to membrane glycoproteins or to cell surface glycolipids. The antigens may also be secreted by tissues as free oligosaccharides or as components of soluble glycoproteins and glycosphingolipids. The blood group O(H) determinant does not generate an immune response, but when modified by addition of either α(1→3)GalNAc (blood group A antigen) or α(1→3)Gal (blood group B antigen), the resultant trisaccharide initiates an immune response (Figure 4).
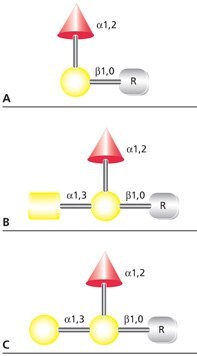
Figure 4.Structures of (A) blood group H(O), (B) blood group A, and (C) blood group B antigens. R represents the hydroxyl-containing amino acid or lipid binding site for the antigen.
T antigen and Tn antigen are O-glycans that have incomplete glycosylation. T antigen (tumor-associated or TF (Thomsen-Fridenreich) antigen) is the Core 1 disaccharide (Gal-β(1→3)GalNAc) that results after desialylation by viral or bacterial neuraminidase. T antigen may also result due to changes in the glycosyltransferases available. Tn antigen is an O-linked GalNAc that is not extended by a glycosyltransferase into a complete core structure. These antigens are not expressed on the surface of normal cells, but are commonly present in cancerous cells and may serve as tumor markers.
Schindler's Disease has been identified as a congenital disorder of glycosylation that affects O-linked glycans and is due to an α-Nacetylgalactosaminidase deficiency.
References
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?