MTOX1013
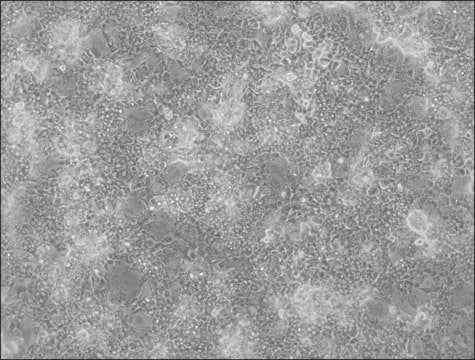
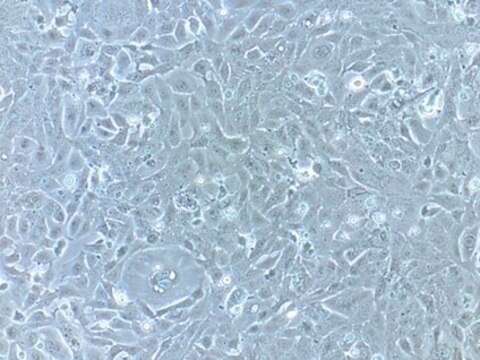
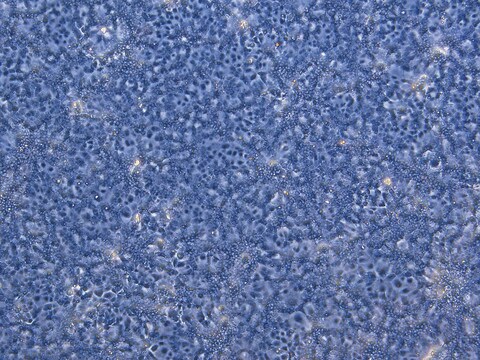
HepaRG™ Cells AHR (-/-)
human female liver (hepatocarcinoma and hepatitis C tumor)
About This Item
Recommended Products
General description
Application
Features and Benefits
- The frame-shift mutation of AHR gene was confirmed by fragment length analysis and DNA sequencing.
- Loss of functionality was confirmed by loss of response to inducers of cytochrome P450 activity.
Quality
Legal Information
Exhibit 2: HepaRG limited use license
Disclaimer
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
MTOX1013-1VL:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service