HPA010023
Anti-HSPA4 antibody produced in rabbit
Prestige Antibodies® Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution
Synonym(s):
Anti-HSP70RY, Anti-Heat shock 70 kDa protein 4, Anti-Heat shock 70-related protein APG-2
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
product line
Prestige Antibodies® Powered by Atlas Antibodies
form
buffered aqueous glycerol solution
species reactivity
human, mouse, rat
technique(s)
immunoblotting: 0.04-0.4 μg/mL
immunofluorescence: 0.25-2 μg/mL
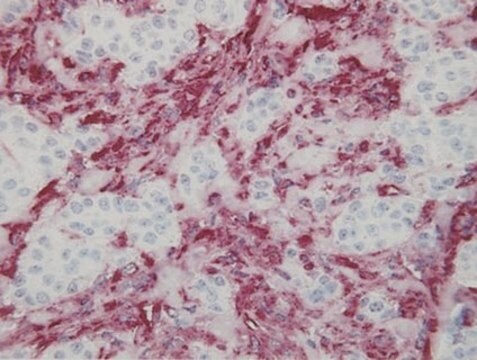
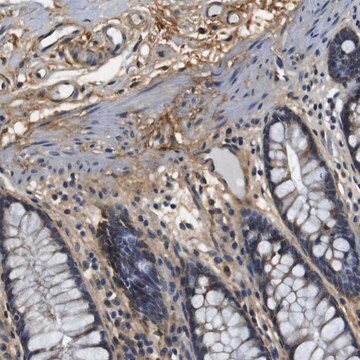
immunohistochemistry: 1:200-1:500
immunogen sequence
FEELGKQIQQYMKIISSFKNKEDQYDHLDAADMTKVEKSTNEAMEWMNNKLNLQNKQSLTMDPVVKSKEIEAKIKELTSTCSPIISKPKPKVEPPKEEQKNAEQNGPVDG
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... HSPA4(3308)
Related Categories
General description
Immunogen
Application
Biochem/physiol Actions
Features and Benefits
Every Prestige Antibody is tested in the following ways:
- IHC tissue array of 44 normal human tissues and 20 of the most common cancer type tissues.
- Protein array of 364 human recombinant protein fragments.
Linkage
Physical form
Legal Information
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
HPA010023-25UL:
HPA010023-100UL:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| HPA010023-100UL | 4061836300609 |
| HPA010023-25UL | 4061842816293 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service