F3627
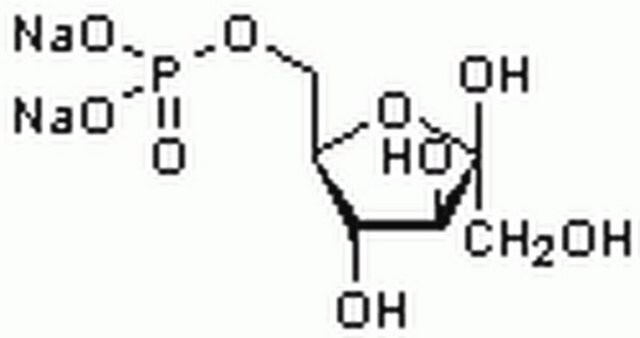
D-Fructose 6-phosphate disodium salt hydrate
≥98%, amorphous powder
Synonym(s):
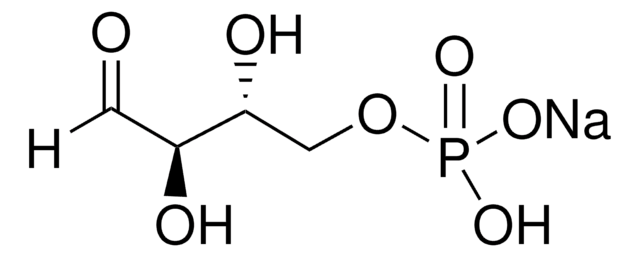
Sodium (2R,3R,4S)-2,3,4,6-tetrahydroxy-5-oxohexyl phosphate
About This Item
Recommended Products
biological source
bacterial (Corynebacterium)
Quality Level
Assay
≥98%
form
amorphous powder
impurities
<0.05 mol % fructose 1,6-diphosphate
<1.5 mol % glucose 6-phosphate
color
white to off-white
solubility
H2O: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
cation traces
Na: 14.6-15.6% (dry basis)
application(s)
agriculture
storage temp.
−20°C
SMILES string
O.[Na+].[Na+].OC[C@@]1(O)O[C@H](COP([O-])([O-])=O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13O9P.2Na.H2O/c7-2-6(10)5(9)4(8)3(15-6)1-14-16(11,12)13;;;/h3-5,7-10H,1-2H2,(H2,11,12,13);;;1H2/q;2*+1;/p-2/t3-,4-,5+,6-;;;/m1.../s1
InChI key
VSCMQICEHMPOEC-HTKRKRNRSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
F3627-10MG-KC:
F3627-1G:4548173370378
F3627-BULK:
F3627PROC:
F3627-10MG:4548173370361
F3627-VAR:
F3627-100MG:4548173370354
F3627-500MG:4548173370385
F3627-PM:
F3627-5G:4548173370392
F3627-CP:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Review the 10 steps of glycolysis in the Embden-Meyerhof-Parnas glycolytic pathway. Easily compare reaction stages and buy the enzymes for your life science research.
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service