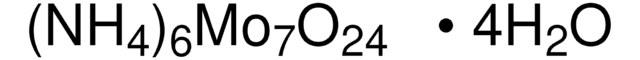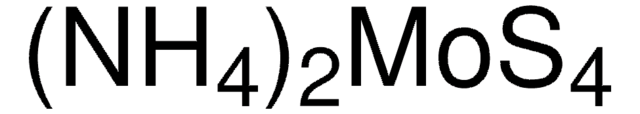431346
Ammonium molybdate tetrahydrate
ACS reagent, 99.98% trace metals basis
Synonym(s):
Ammonium heptamolybdate tetrahydrate, Molybdic acid ammonium salt tetrahydrate
About This Item
Recommended Products
grade
ACS reagent
for inorganic trace analysis
Agency
suitable for SM 5210
Assay
81.0-83.0% MoO3 basis
99.98% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: molybdenum
impurities
≤0.005% insolubles
density
2.498 g/mL at 25 °C (lit.)
anion traces
chloride (Cl-): ≤0.002%
nitrate (NO3-): passes test
phosphate (PO43-): ≤5 ppm
sulfate (SO42-): ≤0.02%
cation traces
Mg and allied cations: ≤0.01%
heavy metals: ≤0.001% (as lead)
SMILES string
N.N.N.N.N.N.O.O.O.O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O=[Mo](=O)=O.O[Mo](O)(=O)=O.O[Mo](O)(=O)=O.O[Mo](O)(=O)=O
InChI
1S/7Mo.6H3N.10H2O.18O/h;;;;;;;6*1H3;10*1H2;;;;;;;;;;;;;;;;;;/q;;;;3*+2;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;;/p-6
InChI key
FIXLYHHVMHXSCP-UHFFFAOYSA-H
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a precursor in the generation of calcium molybdate (CaMoO4) spherulites.
- Synthesis of porous carbon-supported molybdenum dioxide (MoO2).
- Preparation of Iodine-iodide-molybdate solution to determine glucose level by glucose oxidase method.
- Preparation of polymer bulk heterojunction solar cells.
- As a precursor in the synthesis of Mo-N-codoped TiO2 (titanium dioxide).
- Synthesis of MoBi2S5 (molybdenum bismuth sulphide) thin films.
Reagent used for oxidation of sulfides to sulfonyls.
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
431346-250G:4548173940915
431346-VAR:
431346-BULK:
431346-50G:4548173940922
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service