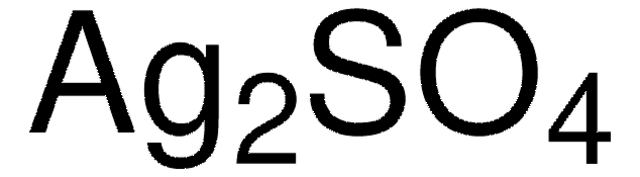28-1320
Silver nitrate
SAJ first grade, ≥99.8%
Synonym(s):
Nitric acid silver(I) salt
About This Item
Recommended Products
grade
SAJ first grade
vapor density
5.8 (vs air)
Assay
≥99.8%
form
solid
availability
available only in Japan
mp
212 °C (dec.) (lit.)
storage temp.
15-25°C
SMILES string
[O-][N+]([O-])=O.[Ag+]
InChI
1S/Ag.NO3/c;2-1(3)4/q+1;-1
InChI key
SQGYOTSLMSWVJD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- In Situ Fabrication of Silver Nanoparticle-Decorated Polymeric Vesicles for Antibacterial Applications.: This article discusses the use of silver nitrate in the synthesis of polymeric vesicles decorated with silver nanoparticles, aimed at enhancing antibacterial properties. This approach represents a significant advancement in the development of targeted antibacterial therapies, showcasing the role of silver nitrate in the field of medical materials science (Zhang et al., 2024).
- Impact of Metal Salt Oxidants and Preparation Technology on Efficacy of Bacterial Cellulose/Polypyrrole Flexible Conductive Fiber Membranes.: This study leverages the oxidizing properties of silver nitrate to enhance the conductivity and flexibility of polymeric fiber membranes. The findings contribute to advancements in wearable electronics and sensors, demonstrating the versatility of silver nitrate in engineering applications (Tao et al., 2024).
- Control of the Hydroquinone/Benzoquinone Redox State in High-Mobility Semiconducting Conjugated Coordination Polymers.: This paper presents the use of silver nitrate in controlling redox states in semiconducting polymers, highlighting its crucial role in the development of high-performance electronic materials. The research underscores the application of silver nitrate in enhancing the electrical properties of novel polymeric materials (Huang et al., 2024).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Ox. Sol. 2 - Repr. 1B - Skin Corr. 1A
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
PRTR
Class I Designated Chemical Substances
FSL
Group 1: Oxidizing solids
Nitrates
Hazardous rank I
1st oxidizing solid
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
28-1320-2-25G-J:
28-1320-5-500G-J:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service