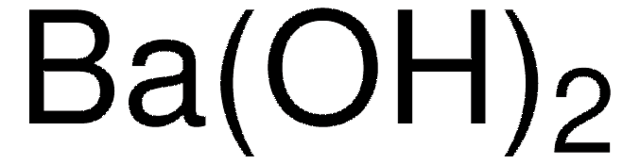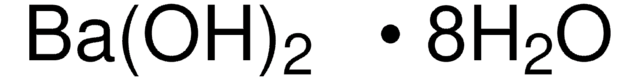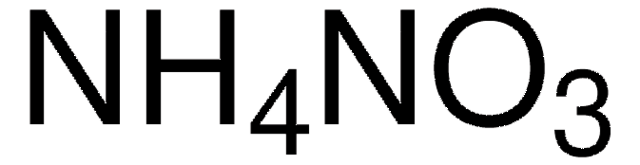1.01737
Barium hydroxide octahydrate
for analysis EMSURE® ACS,ISO,Reag. Ph Eur
Synonym(s):
Barium hydroxide octahydrate, Caustic baryta, Barium oxide hydrate octahydrate
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Agency
reag. ISO
reag. Ph. Eur.
product line
EMSURE®
Assay
≥98.0% (acidimetric)
form
solid
potency
550 mg/kg LD50, oral (Rat)
impurities
≤0.005% Substances insoluble in dilute hydrochloric acid
pH
14 (20 °C in H2O, saturated aqueous solution)
mp
78 °C
density
2.18 g/cm3 at 20 °C
bulk density
900‑1100 kg/m3
anion traces
carbonate (as BaCO3): ≤2.0%
chloride (Cl-): ≤0.001%
sulfide (S2-): ≤0.0005%
cation traces
Ca: ≤0.002%
Fe: ≤0.0005%
K: ≤0.01%
Na: ≤0.01%
Pb: ≤0.001%
Sr: ≤0.5%
heavy metals (as Pb): ≤0.0005%
storage temp.
2-30°C
SMILES string
[Ba+2].[O-H].[O-H].O.O.O.O.O.O.O.O
InChI
1S/Ba.10H2O/h;10*1H2/q+2;;;;;;;;;;/p-2
InChI key
ZUDYPQRUOYEARG-UHFFFAOYSA-L
Application
- Determination of phosphorylated and O-glycosylated sites by chemical targeting (CTID) at ambient temperature.: This study explores the use of chemical targeting to identify phosphorylated and O-glycosylated sites, essential for understanding protein modifications. Barium hydroxide is utilized for its effectiveness in ambient temperature conditions, contributing to more efficient analytical methods (Hathaway, 2007).
- Wet-chemical synthesis of crystalline BaTiO3 from stable chelated titanium complex: formation mechanism and dispersibility in organic solvents.: The research demonstrates the wet-chemical synthesis of BaTiO3, highlighting the formation mechanism and its dispersibility. Barium hydroxide plays a crucial role in stabilizing the chelated titanium complex, making it a valuable component in material synthesis and analytical chemistry (Pramanik et al., 2006).
- Synthesis and analgesic effect of normorphine-3- and -6-glucuronides.: This paper details the synthesis of normorphine glucuronides, where barium hydroxide is used in the synthetic process. The study contributes to the understanding of analgesic properties and the chemical synthesis of pharmacologically relevant compounds (Oguri et al., 1989).
Analysis Note
Substances insoluble in dilute hydrochloric acid: ≤ 0.005 %
Carbonate (as BaCO₃): ≤ 2.0 %
Chloride (Cl): ≤ 0.001 %
Sulphide (S): ≤ 0.0005 %
Heavy metals (as Pb): ≤ 0.0005 %
Ca (Calcium): ≤ 0.002 %
Fe (Iron): ≤ 0.0005 %
K (Potassium): ≤ 0.01 %
Na (Sodium): ≤ 0.01 %
Pb (Lead): ≤ 0.001 %
Sr (Strontium): ≤ 0.5 %
Corresponds to ACS,ISO,Reag. Ph Eur
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PDSCL
Deleterious substance
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
IN21017370500:
1017370000:
IN21017375000:
1017379050:
1017370500:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
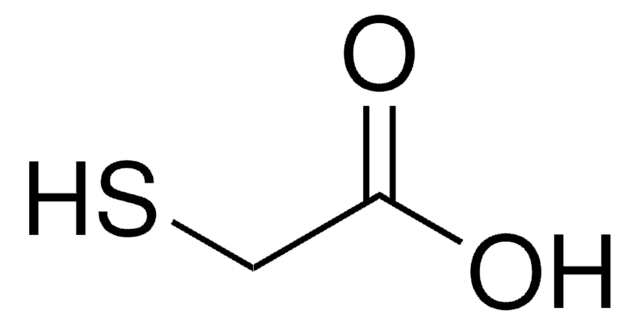
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service