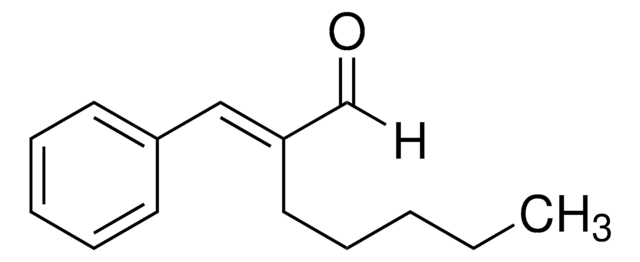
W318101
2-Methoxycinnamaldehyde
natural, 98%, FG
Synonym(s):
o-Methoxycinnamaldehyde
About This Item
Recommended Products
grade
FG
Fragrance grade
Halal
Kosher
natural
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Assay
98%
bp
160-161 °C/12 mmHg (lit.)
mp
44.0-49.0 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
cinnamon; woody; spicy; sweet
SMILES string
[H]C(=O)C=Cc1ccccc1OC
InChI
1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4+
InChI key
KKVZAVRSVHUSPL-GQCTYLIASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Network pharmacology combined with molecular docking and experimental validation to explore the potential mechanism of Cinnamomi ramulus against ankylosing spondylitis.: This study investigates the anti-inflammatory potential of 2-Methoxycinnamaldehyde in Cinnamomi ramulus. Its application extends to novel therapeutic approaches for treating ankylosing spondylitis, demonstrating significant implications for medicinal chemistry and pharmacology (Wei et al., 2023).
- Ramulus Cinnamomi essential oil exerts an anti-inflammatory effect on RAW264.7 cells through N-acylethanolamine acid amidase inhibition.: The study elaborates on the anti-inflammatory activities of 2-Methoxycinnamaldehyde, offering a biochemical pathway that could be exploited in anti-inflammatory drug design (Jia et al., 2023).
- Metabolomics-Driven Exploration of the Antibacterial Activity and Mechanism of 2-Methoxycinnamaldehyde.: This article offers insights into the antibacterial properties of 2-Methoxycinnamaldehyde, using metabolomics to explore its mechanism of action, significant for developments in antimicrobial treatments (Qian et al., 2022).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
235.4 °F
Flash Point(C)
113 °C
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
W318101-10KG-K:4548173338095
W318101-1KG-K:4548173338101
W318101-100G-K:4548173338088
W318101-BULK-K:
W318101-25KG-K:4548173338118
W318101-SAMPLE-K:
W318101-VAR-K:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service