W304522
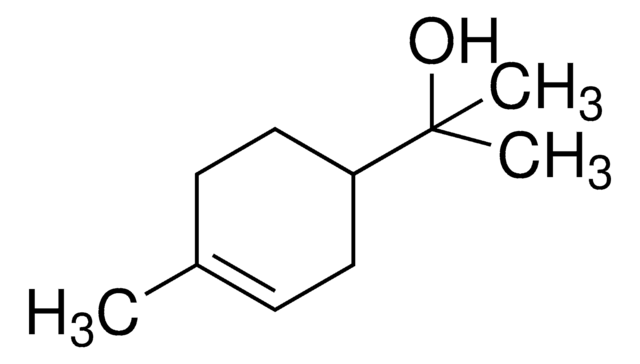
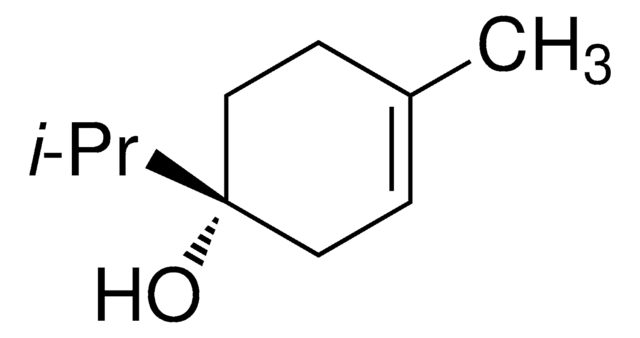
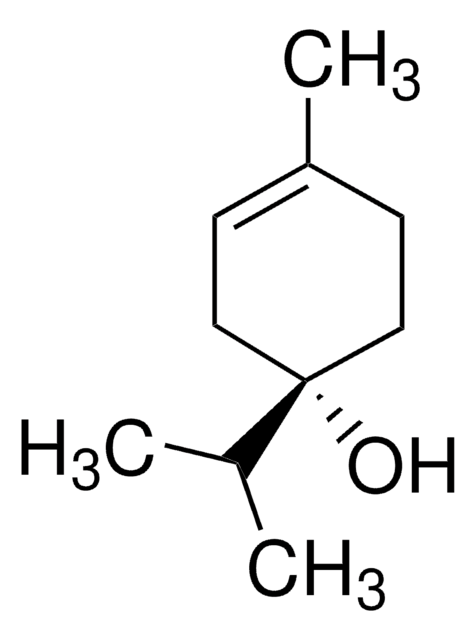
(−)−α-Terpineol
natural, ≥96%, FCC, FG
Synonym(s):
(S)-2-(4-Methyl-3-cyclohexenyl)-2-propanol, (S)-p-Menth-1-en-8-ol
About This Item
Recommended Products
grade
FG
Fragrance grade
Halal
Kosher
natural
Quality Level
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
FDA 21 CFR 178.1010
Assay
≥96%
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1482 (lit.)
bp
217-218 °C (lit.)
mp
31-35 °C (lit.)
density
0.93 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
α-terpineol
greener alternative category
Organoleptic
lilac; citrus; woody; floral; pine
SMILES string
CC1=CC[C@@H](C(O)(C)C)CC1
InChI
1S/C10H18O/c1-8-4-6-9(7-5-8)10(2,3)11/h4,9,11H,5-7H2,1-3H3/t9-/m1/s1
InChI key
WUOACPNHFRMFPN-SECBINFHSA-N
Related Categories
General description
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
FSL
Group 4: Flammable liquids
Type 3 petroleums
Hazardous rank III
Water insoluble liquid
JAN Code
W304522-1KG:
W304522-SAMPLE:
W304522-BULK:
W304522-SAMPLE-K:
W304522-10KG-K:
W304522-BULK-K:
W304522-1KG-K:
W304522-10KG:
W304522-5KG:
W304522-VAR-K:
W304522-5KG-K:
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocols
-(+)-Limonene, purum, ≥98.0% (sum of enantiomers, GC); Geranyl tiglate; α-Terpineol, natural, ≥96%, FCC, FG; Geranyl formate; α-Pinene
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
-α-Bergamotene; β-Bisabolene; α-Terpineol; Neryl acetate; Geranyl acetate; Neral; Geranial
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service