796093
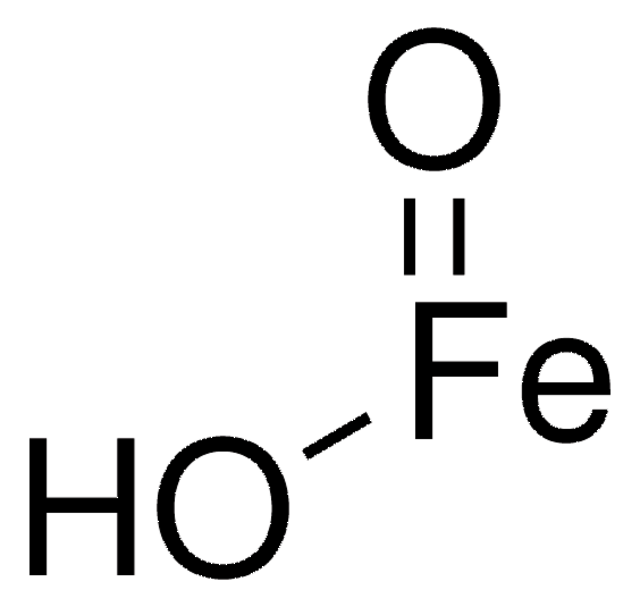
Iron oxide hydroxide
aqueous nanoparticle dispersion, <5 nm (DLS), 20% solids by weight, pH ~3, 99.5% trace metals basis
Synonym(s):
Iron hydroxide oxide, Iron oxyhydroxide
About This Item
Recommended Products
Assay
99.5% trace metals basis
form
dispersion
concentration
20 wt. % in water
particle size
<5 nm (DLS)
pH
3.0±0.5
~3
storage temp.
2-8°C
SMILES string
[Fe+3].[OH-].[O-2]
InChI
1S/Fe.H2O.O/h;1H2;/q+3;;-2/p-1
InChI key
IEECXTSVVFWGSE-UHFFFAOYSA-M
Application
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
796093-VAR:
796093-100ML:
796093-BULK:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Hui Mao explores the use of superparamagnetic iron oxide nanoparticles (INOPs) that offer an alternate contrast-enhancing mechanism.
Professor Yadong Yin (University of California Riverside, USA) examines both direct (thermal decomposition, solvothermal, hydrothermal) and indirect (templated) synthesis methods of magnetite nanocrystals and reviews in detail the landscape of these various synthetic methods for magnetite nanocrystal and their applications in magnetic assembly, magnetic hyperthermia, and Li-Ion batteries.
Electronically, it behaves as a wide band gap (3.2 eV) semiconductor and exhibits memristor properties.2 Optically, TiO2 has high opacity with a very high refractive index3 (>2.4), and it exhibits strong absorbance in the UV range.
Magnetic materials permeate numerous daily activities in our lives. They are essential components of a diversity of products including hard drives that reliably store information on our computers, decorative magnets that keep the shopping list attached to the refrigerator door, electric bicycles that speed our commute to work, as well as wind turbines for conversion of wind energy to electrical power.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service