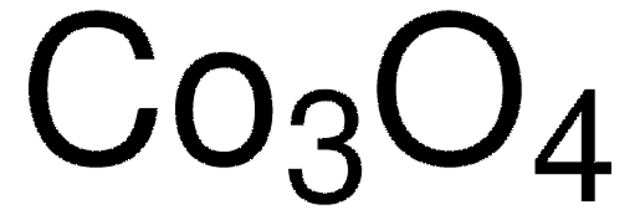544884
Iron(III) oxide
nanopowder, <50 nm particle size (BET)
Synonym(s):
Ferric oxide
About This Item
Recommended Products
description
crystalline (primarily γ)
Quality Level
form
nanopowder
surface area
50-245 m2/g
particle size
<50 nm (BET)
application(s)
battery manufacturing
SMILES string
O=[Fe]O[Fe]=O
InChI
1S/2Fe.3O
InChI key
JEIPFZHSYJVQDO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Features and Benefits
- High theoretical specific capacity
- Biocompatibility
- Ease of coating and modification
- Non-toxicity
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
544884-VAR:
544884-5G:4548173943985
544884-BULK:
544884-25G:4548173943978
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Innovation in dental restorative materials is driven by the need for biocompatible and natural-appearing restoration alternatives. Conventional dental materials like amalgam and composite resins have inherent disadvantages.
Currently, magnetic nanoparticles (MNPs) are attracting a lot of attention because of the possibility of many novel applications, especially in biomedical research.
Graphene is a unique two-dimensional (2D) structure of monolayer carbon atoms packed into a dense honeycomb crystal that has attracted great interest due to its diverse and fascinating properties.
Professor Hui Mao explores the use of superparamagnetic iron oxide nanoparticles (INOPs) that offer an alternate contrast-enhancing mechanism.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service