773263
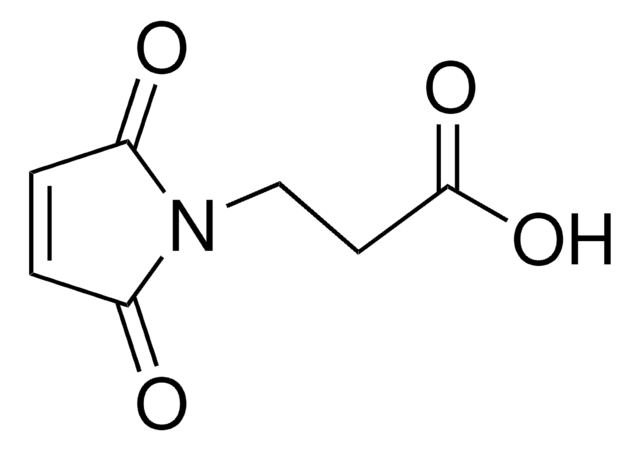
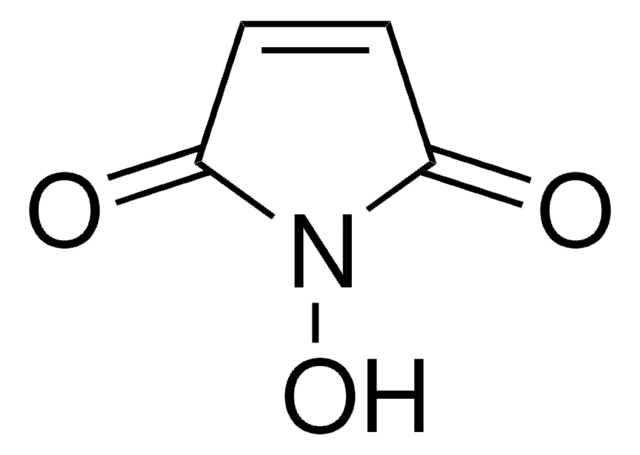
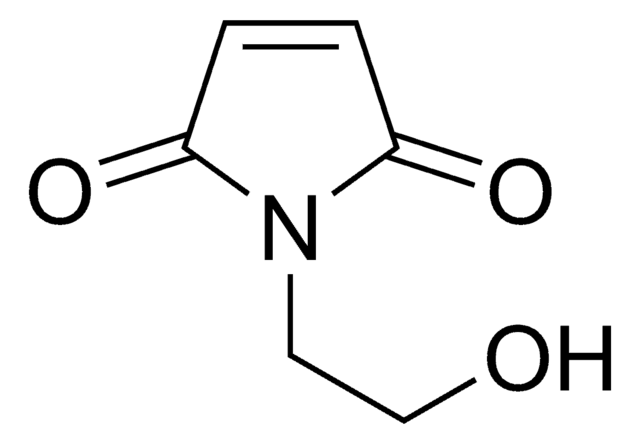
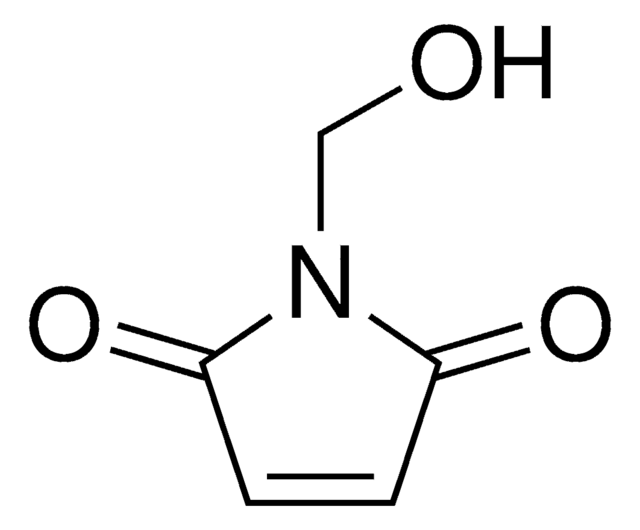
N-(2-Hydroxyethyl)maleimide
97%
Synonym(s):
1-(2-Hydroxyethyl)-pyrrole-2,5-dione, 1-(2-Hydroxyethyl)maleimide, N-(Ethanol)maleimide, HEMI
About This Item
Recommended Products
Assay
97%
form
solid
reaction suitability
reagent type: cross-linking reagent
mp
66-67 °C (lit.)
67-74 °C
functional group
hydroxyl
maleimide
storage temp.
2-8°C
SMILES string
O=C(N1CCO)C=CC1=O
InChI
1S/C6H7NO3/c8-4-3-7-5(9)1-2-6(7)10/h1-2,8H,3-4H2
InChI key
AXTADRUCVAUCRS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- In the synthesis of (EFA)-based multifunctional oligoester resins with maleimides as end groups by reacting with 9,10-epoxy-18-hydroxyoctadecanoic acid (EFA) and dimethyl adipate catalyzed by Candida antarctica lipase B (CalB).
- As an initiator in ring-opening polymerization of δ-valerolactone catalyzed by trifluoromethanesulfonimide.
- In one-pot synthesis of bio-based polyurethane-imides by reacting with castor oil, eleostearic acid diethanol amide and isophorone diisocyanate.
propane (BMEP)], a protein cross-linking reagent with potential application in constructing immunotoxins. It may also be used in preparing thermoresponsive self-healing polyurethanes with the shape-memory property.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
JAN Code
773263-250MG:
773263-VAR:
773263-1G:
773263-BULK:
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service