T8516
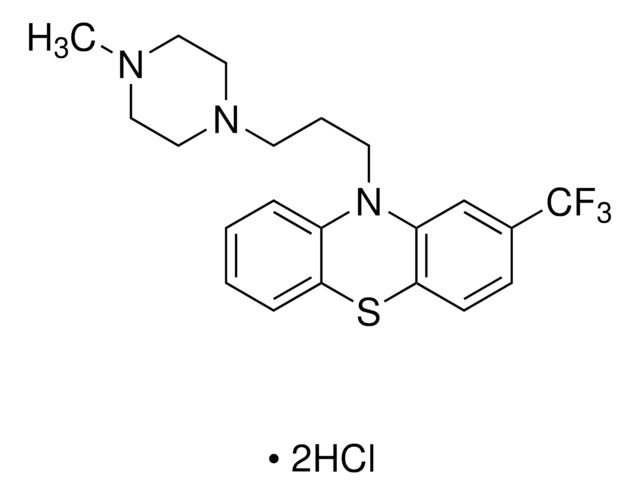
Trifluoperazine dihydrochloride
≥99%, powder
Sinonimo/i:
10-[3-(4-Methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)-10H-phenothiazine dihydrochloride
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥99%
Stato
powder
Colore
white to off-white
Punto di fusione
243 °C (dec.) (lit.)
Solubilità
ethanol: soluble 5 mg/mL
H2O: soluble 50 mg/mL, clear, colorless to yellow
DMSO: soluble
Temperatura di conservazione
−20°C
Stringa SMILE
Cl[H].Cl[H].CN1CCN(CCCN2c3ccccc3Sc4ccc(cc24)C(F)(F)F)CC1
InChI
1S/C21H24F3N3S.2ClH/c1-25-11-13-26(14-12-25)9-4-10-27-17-5-2-3-6-19(17)28-20-8-7-16(15-18(20)27)21(22,23)24;;/h2-3,5-8,15H,4,9-14H2,1H3;2*1H
BXDAOUXDMHXPDI-UHFFFAOYSA-N
Informazioni sul gene
human ... DRD2(1813) , DRD3(1814) , DRD4(1815) , HTR2A(3356) , HTR2C(3358)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- to inhibit clathrin-dependent endocytosis
- as a calmodulin kinase antagonist in cultured Aplysia californica neurons
- as a plasma membrane Ca2+-ATPase (PMCA) inhibitor in mouse duodenal tissues to block the transcellular active calcium flux
Azioni biochim/fisiol
Caratteristiche e vantaggi
Nota sulla preparazione
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Muta. 2 - STOT RE 1 - STOT SE 3
Organi bersaglio
Central nervous system, Eyes
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.