T0202
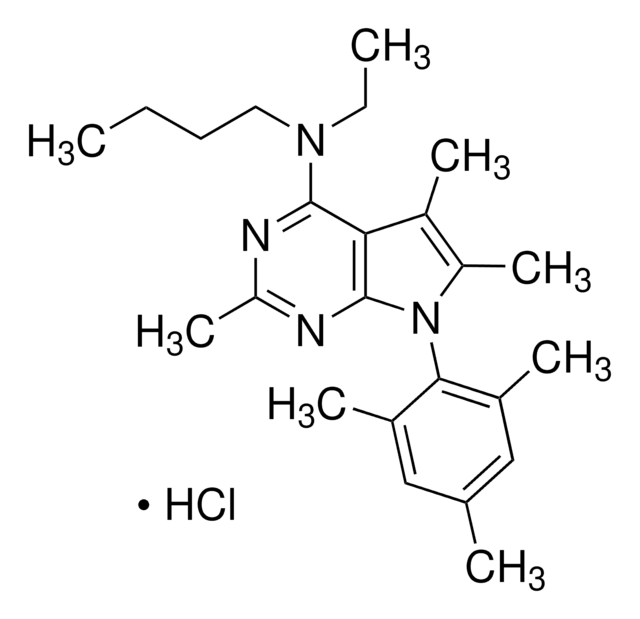
Tocainide
≥98% (HPLC), solid
Sinonimo/i:
2-Amino-N-(2,6-dimethylphenyl)propanamide
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥98% (HPLC)
Stato
solid
Condizioni di stoccaggio
desiccated
under inert gas
Colore
white
Solubilità
DMSO: >20 mg/mL
H2O: ≥5 mg/mL
Ideatore
AstraZeneca
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl[H].CC(N)C(=O)Nc1c(C)cccc1C
InChI
1S/C11H16N2O.ClH/c1-7-5-4-6-8(2)10(7)13-11(14)9(3)12;/h4-6,9H,12H2,1-3H3,(H,13,14);1H
AMZACPWEJDQXGW-UHFFFAOYSA-N
Informazioni sul gene
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Voltage-gated sodium channels are present in most excitable cell membranes and play an important role in generating action potentials.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.