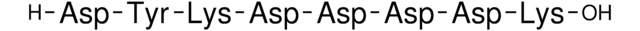SRP2120
RNA Polymerase II Peptide
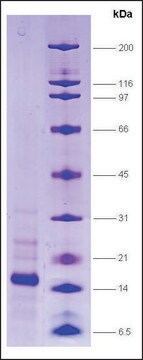
≥70% (SDS-PAGE), human recombinant, expressed in E. coli, GST tagged
Sinonimo/i:
POLRA, RPB1, RPO2, RPOL2, hRPB220
About This Item
Prodotti consigliati
product name
RNA Polymerase II, C-terminal domain, GST tagged human, recombinant, expressed in E. coli, ≥70% (SDS-PAGE)
Origine biologica
human
Ricombinante
expressed in E. coli
Saggio
≥70% (SDS-PAGE)
Forma fisica
frozen liquid
PM
~68.1 kDa
Confezionamento
pkg of 10 μg
Condizioni di stoccaggio
avoid repeated freeze/thaw cycles
Colore
clear colorless
N° accesso NCBI
N° accesso UniProt
Condizioni di spedizione
dry ice
Temperatura di conservazione
−70°C
Informazioni sul gene
human ... POLR2A(5430)
Categorie correlate
Azioni biochim/fisiol
Stato fisico
Nota sulla preparazione
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.