SAE0093
Beta-1,4-galactosyltransferase 1
B4GALT1 human recombinant, expressed in HEK 293 cells, 2000 units/mg protein
Sinonimo/i:
Beta-1,4-GalTase 1, Beta4Gal-T1, UDP-Gal:beta-GlcNAc beta-1,4-galactosyltransferase 1, UDP-galactose:beta-N-acetylglucosamine beta-1,4-galactosyltransferase 1, b4Gal-T1
About This Item
Prodotti consigliati
Ricombinante
expressed in HEK 293 cells
Saggio
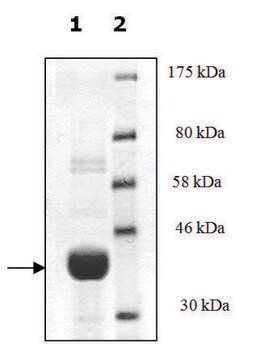
95% (SDS-PAGE)
Stato
lyophilized powder
Attività specifica
2000 units/mg protein
Condizioni di spedizione
ambient
Temperatura di conservazione
−20°C
Descrizione generale
Applicazioni
Azioni biochim/fisiol
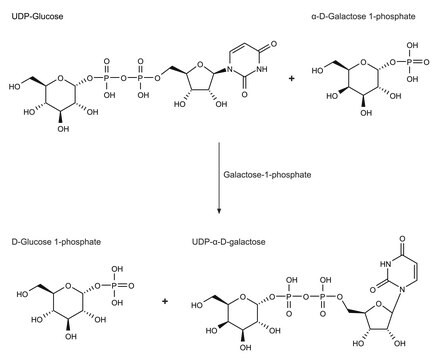
A major function of B4GALT1 is the addition of β(1→4) linked galactose residues to oligosaccharide acceptors with terminal N-acetylglucosamine residues. This is a late elongation step in the N-glycan processing pathway.B4GALT1 enzymatic activity is widely distributed in the vertebrate kingdom, in both mammals and non-mammals, including avians and amphibians.B4GALT1 enzymatic activity has also been demonstrated in a subset of plants which diverged from animals an estimated 1 billion years ago.B4GALT1 interacts with α-lactalbumin (LA), a protein expressed in the mammary gland during lactation, to form the lactose synthase (LS) complex that transfers galactose from UDP-α-D-Gal to glucose, producing the lactose secreted in milk.Defects in B4GALT1 are the cause of congenital disorder of glycosylation type 2D (CDG2D).Glomerular B4GALT1 expression has been found to be increased in IgA nephropathy. IgA binding and IgA-induced mesangial cell phosphorylation of spleen tyrosine kinase and IL-6 synthesis were inhibited by a panel of β(1→4) galactosyltransferase-specific antibodies, which suggests that IgA binds to the catalytic domain of β(1→4) galactosyltransferase.
Definizione di unità
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Glycosyltransferases were initially considered to be specific for a single glycosyl donor and acceptor, which led to the one enzyme-one linkage concept. Subsequent observations have refuted the theory of absolute enzymatic specificity by describing the transfer of analogs of some nucleoside mono- or diphosphate sugar donors.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.