L6004
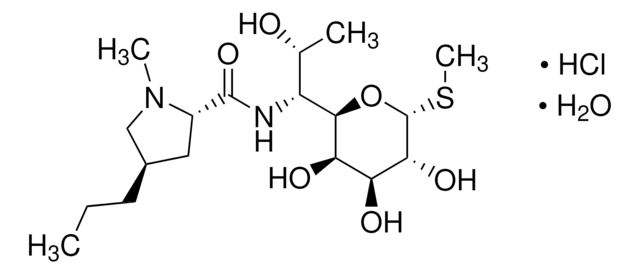
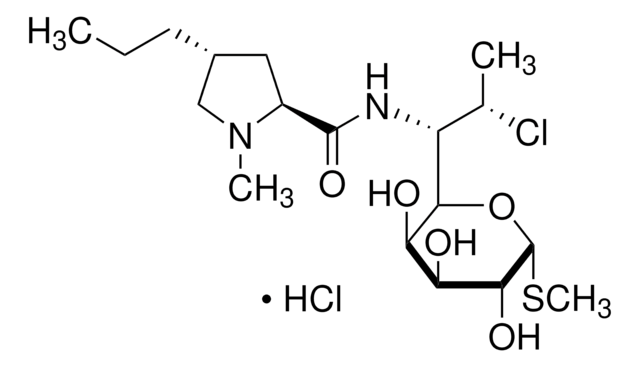
Lincomycin hydrochloride
≥90% (TLC)
Sinonimo/i:
Epilincomycin, Lincomycin HCL, Lincocin hydrochloride, Methyl 6,8-dideoxy-6-(1-methyl-4-propyl-2-pyrrolidinecarboxamido)-1-thio-D-erythro-α-D-galactooctopyranoside hydrochloride
About This Item
Prodotti consigliati
Saggio
≥90% (TLC)
Potenza
800-900 units per mg
Colore
white to faint yellow
Solubilità
H2O: soluble 50 mg/mL
Spettro attività antibiotica
Gram-positive bacteria
Modalità d’azione
protein synthesis | interferes
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl.CCC[C@@H]1C[C@H](N(C)C1)C(=O)N[C@H]([C@@H](C)O)[C@H]2O[C@H](SC)[C@H](O)[C@@H](O)[C@H]2O
InChI
1S/C18H34N2O6S.ClH/c1-5-6-10-7-11(20(3)8-10)17(25)19-12(9(2)21)16-14(23)13(22)15(24)18(26-16)27-4;/h9-16,18,21-24H,5-8H2,1-4H3,(H,19,25);1H/t9-,10-,11+,12-,13+,14-,15-,16-,18-;/m1./s1
POUMFISTNHIPTI-BOMBIWCESA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Antimicrobial spectrum: Lincomycin hydrochloride is effective against gram-positive bacteria.
Nota sulla preparazione
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.