K0133
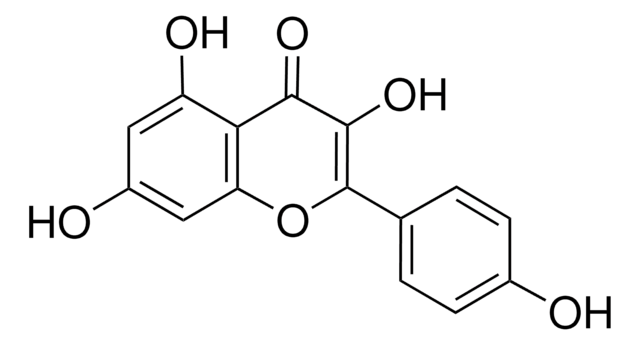
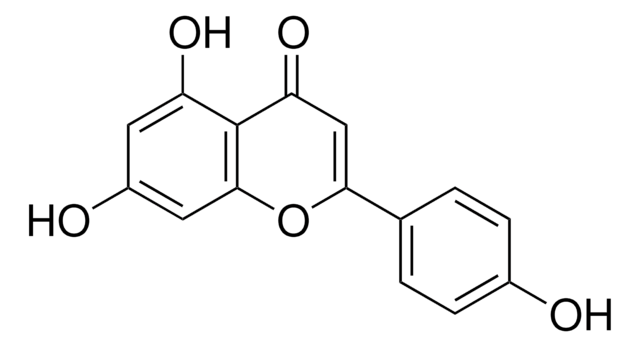
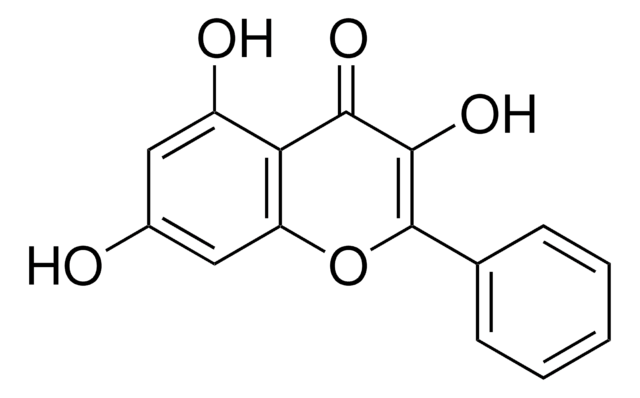
Kaempferol
≥90% (HPLC), powder
Sinonimo/i:
3,4′,5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, Robigenin
About This Item
Prodotti consigliati
Origine biologica
synthetic
Livello qualitativo
Saggio
≥90% (HPLC)
Forma fisica
powder
Condizioni di stoccaggio
protect from light
Colore
yellow
Punto di fusione
277 °C
Solubilità
ethanol: 20 mg/mL
DMSO: 50 mg/mL
Temperatura di conservazione
room temp
Stringa SMILE
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
IYRMWMYZSQPJKC-UHFFFAOYSA-N
Informazioni sul gene
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- to check its potential effect as an antioxidant and neuroprotective agent against rotenone-induced Parkinson′s disease (PD) model in SH-S5Y5 cells
- to test its anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammatory injury in human aortic endothelial cells (HAECs)
- to study its apoptosis sensitizing effect on non-small cell lung cancer (NSCLC) cells by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2)
Azioni biochim/fisiol
Confezionamento
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.