D7408
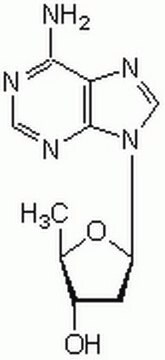
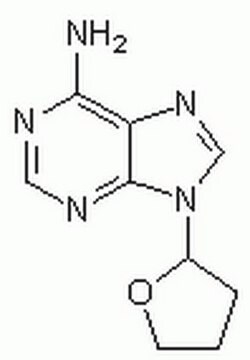
2′,5′-Dideoxyadenosine
≥95% (HPLC), solid
Sinonimo/i:
2ʹ,5ʹ-dd-Ado, NSC 95943
About This Item
Prodotti consigliati
Saggio
≥95% (HPLC)
Stato
solid
Colore
white
Solubilità
DMSO: soluble
Temperatura di conservazione
−20°C
Stringa SMILE
C[C@H]1O[C@H](C[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H13N5O2/c1-5-6(16)2-7(17-5)15-4-14-8-9(11)12-3-13-10(8)15/h3-7,16H,2H2,1H3,(H2,11,12,13)/t5-,6+,7-/m1/s1
FFHPXOJTVQDVMO-DSYKOEDSSA-N
Informazioni sul gene
rat ... Adcy2(81636)
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Ricostituzione
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Contenuto correlato
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.