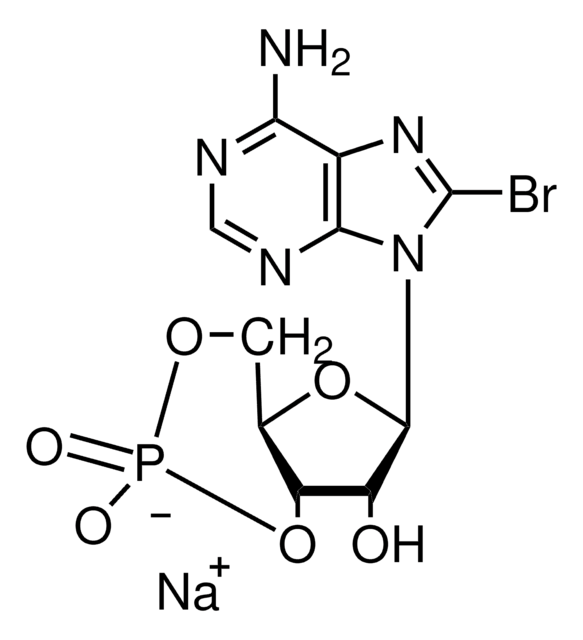
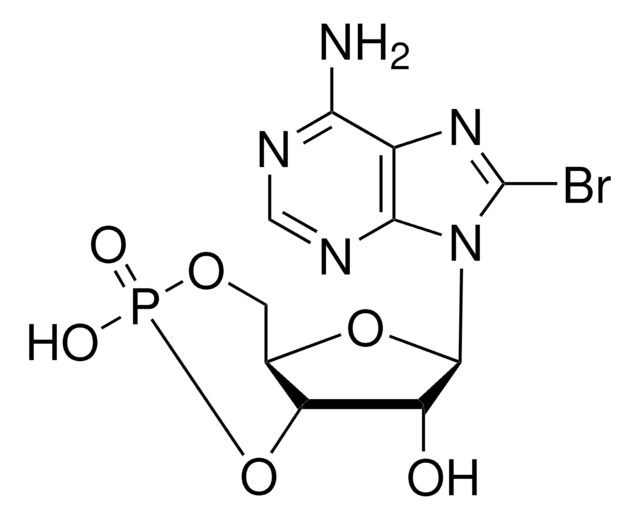
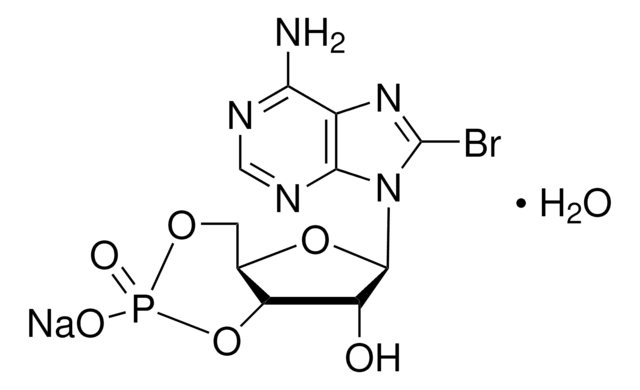
B3131
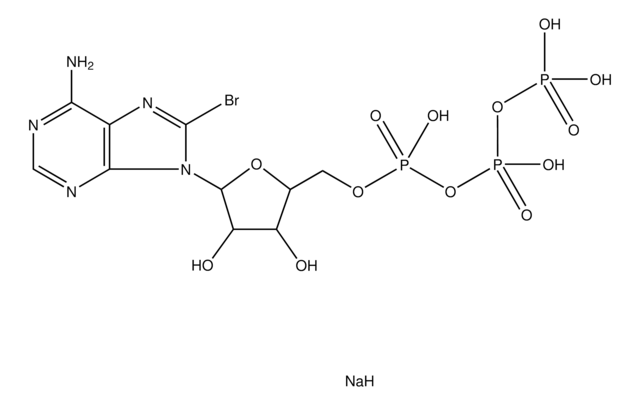
8-Bromoadenosine 5′-monophosphate
≥98%
Sinonimo/i:
8-Br-AMP
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H13BrN5O7P
Numero CAS:
Peso molecolare:
426.12
Numero MDL:
Codice UNSPSC:
41106305
ID PubChem:
NACRES:
NA.51
Prodotti consigliati
Origine biologica
synthetic (organic)
Saggio
≥98%
Stato
powder
Solubilità
water: 100 mg/mL, clear, colorless
Temperatura di conservazione
−20°C
Stringa SMILE
Nc1ncnc2n(C3OC(COP(O)(O)=O)C(O)C3O)c(Br)nc12
InChI
1S/C10H13BrN5O7P/c11-10-15-4-7(12)13-2-14-8(4)16(10)9-6(18)5(17)3(23-9)1-22-24(19,20)21/h2-3,5-6,9,17-18H,1H2,(H2,12,13,14)(H2,19,20,21)
DNPIJKNXFSPNNY-UHFFFAOYSA-N
Categorie correlate
Applicazioni
8-Bromoadenosine 5′′-monophosphate (8-Br-AMP) is an analogue of 5′-AMP useful for receptor mapping studies; as a starting structure for 8-modified 5′-AMP derivatives and for synthesis of poly-8-bromoriboadenylic acid.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
K Lesiak et al.
Biochemical and biophysical research communications, 126(2), 917-921 (1985-01-31)
When added to extracts of mouse L cells containing ATP and an energy regenerating system, the 5'-diphosphate of 2-5A core, pp5'A2'p5'A2'p5'A, as well as a bromoadenylate analog, pp5' (br8A)2'p5'(br8A)2'p5'(br8A), can be phosphorylated to the corresponding 5'-triphosphate, ppp5'A2'p5'A2'p5'A and ppp5'(br8A)2'p5'(br8A)2'p5(br8A), respectively.
E N Kanaya et al.
Biochemistry, 23(18), 4219-4225 (1984-08-28)
Introduction of the bulky 8-bromo substituent into adenine residues of polynucleotides has strikingly different consequences in the deoxy- and ribopolynucleotide series. Poly(r8BrA) was found in earlier studies to form a very stable double-helical self-structure but not to undergo interaction with
Rongkuan Hu et al.
PloS one, 8(8), e73527-e73527 (2013-08-24)
This study is the first to demonstrate that shizukaol D, a natural compound isolated from Chloranthusjaponicus, can activate AMP- activated protein kinase (AMPK), a key sensor and regulator of intracellular energy metabolism, leading to a decrease in triglyceride and cholesterol
J O Folayan et al.
Journal of biochemistry, 96(4), 1297-1301 (1984-10-01)
Poly-8-bromoriboadenylic acid was synthesized by the bromination of adenosine-5'-monophosphate to yield 8-bromoadenosine-5'-monophosphate which on conversion to the 5'-diphosphate form was polymerized by polynucleotide phosphorylase (PNPase). The polymer formed a 1:1 hybrid with polyribouridylic acid and the hybrid was found to
Gijs Teklenburg et al.
PloS one, 5(4), e10258-e10258 (2010-04-28)
Pregnancy is widely viewed as dependent upon an intimate dialogue, mediated by locally secreted factors between a developmentally competent embryo and a receptive endometrium. Reproductive success in humans is however limited, largely because of the high prevalence of chromosomally abnormal
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.