B178
Butein
solid
Sinonimo/i:
1-(2,4-Dihydroxyphenyl)-3-(3,4-dihydroxyphenyl)-2-propen-1-one, 2′,3,4,4′-Tetrahydroxychalcone
About This Item
Prodotti consigliati
Stato
solid
Colore
yellow
Solubilità
DMSO: >50 mg/mL
H2O: insoluble
Stringa SMILE
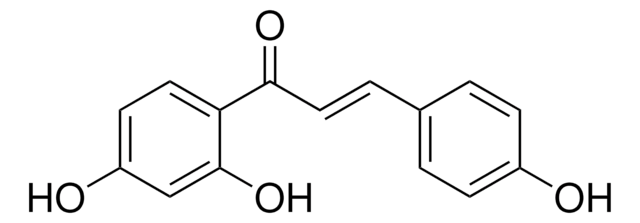
OC1=CC(O)=C(C(/C=C/C2=CC(O)=C(O)C=C2)=O)C=C1
InChI
1S/C15H12O5/c16-10-3-4-11(14(19)8-10)12(17)5-1-9-2-6-13(18)15(20)7-9/h1-8,16,18-20H/b5-1+
AYMYWHCQALZEGT-ORCRQEGFSA-N
Informazioni sul gene
rat ... Alox5(25290)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenza
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.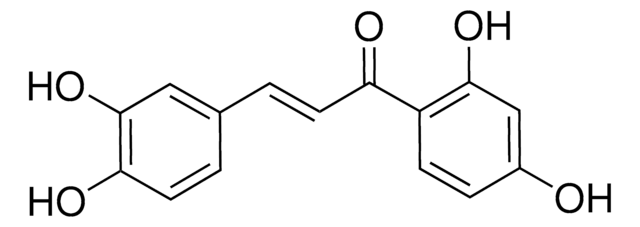



![[Arg8]-Vasopressin solution Grade VI (synthetic), ~100 IU/mL in 0.9% NaCl](/deepweb/assets/sigmaaldrich/product/structures/326/242/dede8c26-cf73-4a28-a5d9-1d57c673cf0e/640/dede8c26-cf73-4a28-a5d9-1d57c673cf0e.png)