ABK0007
HercBridge ELISA Kit
Sinonimo/i:
Protein Conformational Array Herceptin Biosimilar, Protein Conformational Array Trastuzumab
About This Item
Prodotti consigliati
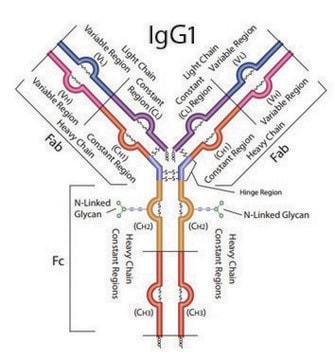
Forma dell’anticorpo
IgG fraction of antiserum
Reattività contro le specie
human (Human IgG)
Condizioni di spedizione
wet ice
Temperatura di conservazione
2-8°C
Descrizione generale
Applicazioni
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Articoli
Monoclonal antibodies (mAbs) are the fastest growing class of human therapeutics within the field of biologics, having predicted worldwide sales of $125 billion by 2020 based on current approval rates.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.