A1289
Apamin
from bee venom, ≥95% (HPLC)
About This Item
Prodotti consigliati
Origine biologica
bee venom
Livello qualitativo
Saggio
≥95% (HPLC)
Stato
powder
PM
2027.34 g/mol
tecniche
inhibition assay: suitable
Nota sulla sequenza
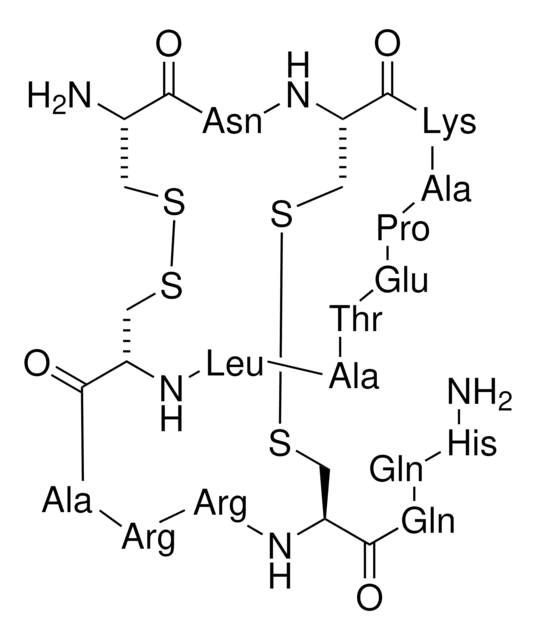
Cys-Asn-Cys-Lys-Ala-Pro-Glu-Thr-Ala-Leu-Cys-Ala-Arg-Arg-Cys-Gln-Gln-His-NH2 [Disulfide Bridges: 1-11, 3-15]
N° accesso UniProt
Temperatura di conservazione
−20°C
Stringa SMILE
CC(C)C[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]2CCC[N@H]2C(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]3CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CSSC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N3)NC1=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc4cnc[nH]4)C(N)=O)[C@@H](C)O
InChI
1S/C79H131N31O24S4/c1-35(2)26-49-70(127)107-51-31-136-135-30-41(81)63(120)105-50(28-57(84)114)71(128)108-53(73(130)99-42(12-7-8-22-80)64(121)96-38(5)77(134)110-25-11-15-54(110)75(132)102-47(18-21-58(115)116)69(126)109-59(39(6)111)76(133)95-37(4)62(119)104-49)33-138-137-32-52(106-66(123)44(14-10-24-92-79(88)89)98-65(122)43(13-9-23-91-78(86)87)97-61(118)36(3)94-72(51)129)74(131)101-45(16-19-55(82)112)67(124)100-46(17-20-56(83)113)68(125)103-48(60(85)117)27-40-29-90-34-93-40/h29,34-39,41-54,59,111H,7-28,30-33,80-81H2,1-6H3,(H2,82,112)(H2,83,113)(H2,84,114)(H2,85,117)(H,90,93)(H,94,129)(H,95,133)(H,96,121)(H,97,118)(H,98,122)(H,99,130)(H,100,124)(H,101,131)(H,102,132)(H,103,125)(H,104,119)(H,105,120)(H,106,123)(H,107,127)(H,108,128)(H,109,126)(H,115,116)(H4,86,87,91)(H4,88,89,92)/t36-,37-,38-,39+,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1
YVIIHEKJCKCXOB-STYWVVQQSA-N
Informazioni sul gene
rat ... Kcnn1(54261)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Amino Acid Sequence
Descrizione generale
Apamin is a bee venom component and is strongly basic in nature. It has 18 amino acid residues and two disulphide bonds.
Applicazioni
- As a selective inhibitor of small conductance (SKCa) channels in HEK cells.
- To inhibit endothelium-derived relaxing factor (EDHF) mediated responses.
- To block small-conductance Ca2+-activated K+current (/SK) in electrophysiological studies in hyperstriatum ventrale, pars caudalis (HVc) neurons.
- as a standard for determination of content and compositions of bee venom by high-performance liquid chromatography (HPLC).
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.