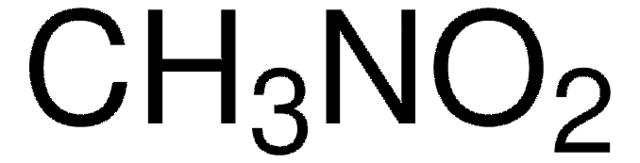270423
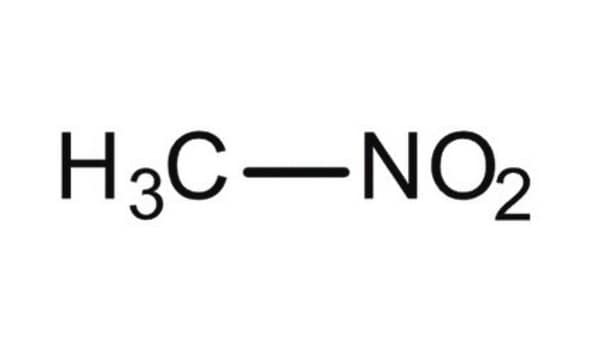
Nitromethane
suitable for HPLC, ≥96%
About This Item
Prodotti consigliati
Grado
HPLC grade
Densità del vapore
2.1 (vs air)
Tensione di vapore
2.7 mmHg
Saggio
≥96%
Stato
liquid
Temp. autoaccensione
784 °F
Limite di esplosione
7.3 %, 33 °F
tecniche
HPLC: suitable
Impurezze
<0.030% water
Indice di rifrazione
n20/D 1.382 (lit.)
pH
6.4 (20 °C, 0.01 g/L)
P. ebollizione
101.2 °C (lit.)
Punto di fusione
−29 °C (lit.)
Densità
1.127 g/mL at 25 °C (lit.)
λ
H2O reference
Assorbanza UV
λ: 380 nm Amax: 1.00
λ: 386 nm Amax: 0.50
λ: 395 nm Amax: 0.20
λ: 400 nm Amax: 0.10
λ: 405 nm Amax: 0.05
λ: 430-700 nm Amax: 0.01
applicazioni
food and beverages
Stringa SMILE
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
LYGJENNIWJXYER-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Asymmetric aza-Henry reaction toward trifluoromethyl β-nitroamines and biological investigation of their adamantane-type derivatives.: This study used nitromethane in asymmetric aza-Henry reactions to synthesize trifluoromethyl β-nitroamines, which were further investigated for their biological properties, showcasing the potential of nitromethane in advanced synthetic chemistry (Ren et al., 2024).
- Effect of Temperature on the Liquid Bridging Force while Maintaining Physical Stability in Solid-Liquid Mixed Fuel.: Nitromethane was analyzed in this research to understand its role in the stability of solid-liquid mixed fuels under varying temperatures, providing insights into the optimization of fuel formulations (Zhang et al., 2024).
- Generation of New Synthons for Synthesis Through Activation of Nitromethane.: This research demonstrated the activation of nitromethane to generate new synthons for synthetic applications, highlighting its versatility and importance in creating novel chemical entities (Wang et al., 2024).
- Towards Chemoenzymatic Syntheses of Both Enantiomers of Phosphoemeriamine.: The study explored the use of nitromethane in chemoenzymatic syntheses, enabling the production of both enantiomers of phosphoemeriamine, an important compound in chemical biology (Kiełbasiński et al., 2024).
- Rationally introducing non-canonical amino acids to enhance catalytic activity of LmrR for Henry reaction.: Nitromethane was employed in this study to investigate the enhancement of catalytic activity in the Henry reaction through the introduction of non-canonical amino acids, demonstrating its significance in enzyme catalysis research (Wang et al., 2024).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
Codice della classe di stoccaggio
4.1A - Other explosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
95.0 °F - closed cup
Punto d’infiammabilità (°C)
35 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Protocolli
GC Analysis of Class 2 Residual Solvents on OVI-G43
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.