PHR1880
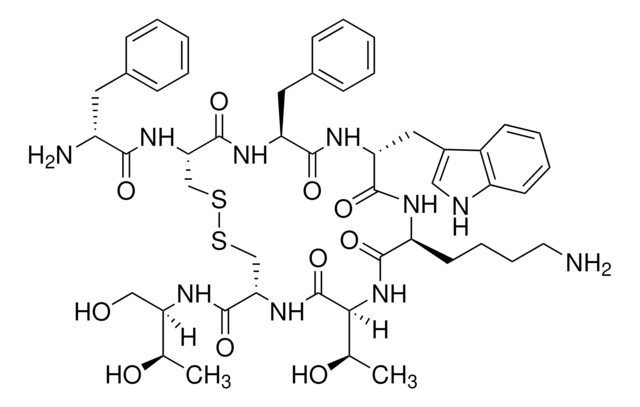
Octreotide Acetate
Pharmaceutical Secondary Standard; Certified Reference Material
Sinonimo/i:
Octreotide acetate salt
About This Item
Prodotti consigliati
Grado
certified reference material
pharmaceutical secondary standard
Livello qualitativo
agenzia
traceable to USP 1477604
Famiglia di API
octreotide
Forma fisica
powder
CdA
current certificate can be downloaded
Confezionamento
pkg of 10 mg
applicazioni
pharmaceutical
Temperatura di conservazione
-10 to -25°C
InChI
1S/C49H66N10O10S2.2C2H4O2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41;2*1-2(3)4/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64);2*1H3,(H,3,4)/t28-,29?,34?,36?,37?,38?,39-,40?,41?,42?;;/m1../s1
QWFYIFWTVZFPRY-AARKYNAGSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
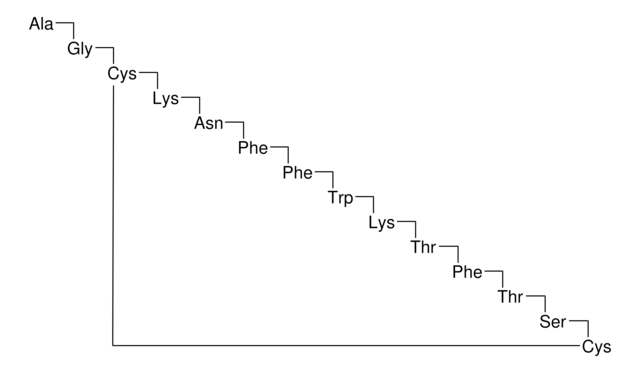
Octreotide belongs to the group of synthetic cyclic octapeptides, known for its selectivity towards inhibiting the growth hormone. It binds to the somatostatin receptor 2 (SSTR2), and thereby prevents the secretion pathways of growth hormone. Hence it is used in the treatment of diseases caused by overproduction of growth hormone, such as acromegaly.
Applicazioni
- Octreotide acetate analysis of amino acids including hydrolysis, derivatization of released amino acids with 4-N,N-dimethylaminoazobenzene-4ʹ-sulfonyl chloride (DABS-Cl), and finally their reversed phase-high performance liquid chromatography (RP-HPLC) determination
- Development of a quantitative nuclear magnetic resonance (1H-qNMR) based method for the estimation of octreotide acetate in bulk drug
- Determination of octreotide acetate in pharmaceutical formulations using a stability-indicating capillary zone electrophoresis method (CZE)
- Estimation of octreotide acetate from a peptide-based hydrogel using an ultra-high performance liquid chromatographic method in combination with photo-diode array detection (PDA), following the quality-by-design (QbD) approach
Risultati analitici
Nota a piè di pagina
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.