E0774
Erythromycin
meets USP testing specifications
Sinonimo/i:
Erythromycin A
About This Item
Prodotti consigliati
agenzia
USP/NF
meets USP testing specifications
Livello qualitativo
Forma fisica
solid
Attività ottica
[α]/D -78 to --71°
Solubilità
ethanol: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
Spettro attività antibiotica
Gram-negative bacteria
Gram-positive bacteria
applicazioni
pharmaceutical (small molecule)
Modalità d’azione
protein synthesis | interferes
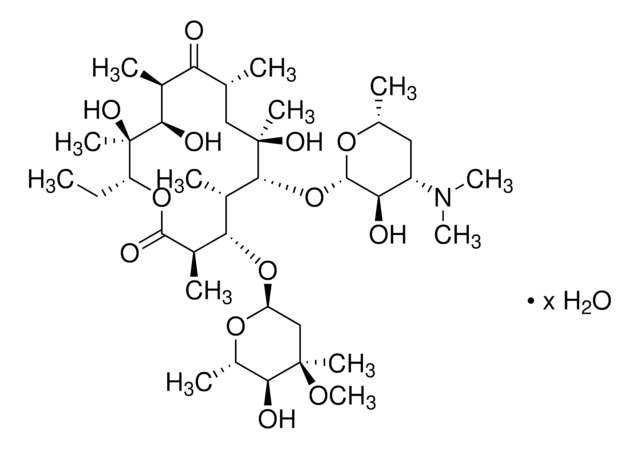
Stringa SMILE
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Informazioni sul gene
human ... ABCB1(5243) , CYP3A4(1576) , MLNR(2862)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Avvertenza
Nota sulla preparazione
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Protein synthesis is a complex, multi-step process involving many enzymes as well as conformational alignment. However, the majority of antibiotics that block bacterial protein synthesis interfere with the processes at the 30S subunit or 50S subunit of the 70S bacterial ribosome.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.