80757
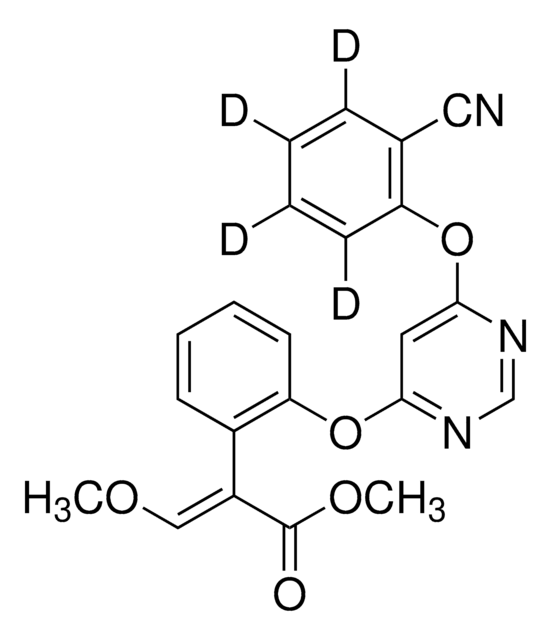
Propamocarb-(propyl-d7)
PESTANAL®, analytical standard
Sinonimo/i:
Propamocarb-d7
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C9D7H13N2O2
Peso molecolare:
195.31
Numero MDL:
Codice UNSPSC:
41116107
ID PubChem:
NACRES:
NA.24
Prodotti consigliati
Grado
analytical standard
Livello qualitativo
Nome Commerciale
PESTANAL®
Saggio
≥98.0% (GC)
Durata
limited shelf life, expiry date on the label
applicazioni
agriculture
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
CN(C)CCCNC(OC([2H])([2H])C([2H])([2H])C([2H])([2H])[2H])=O
InChI
1S/C9H20N2O2/c1-4-8-13-9(12)10-6-5-7-11(2)3/h4-8H2,1-3H3,(H,10,12)/i1D3,4D2,8D2
WZZLDXDUQPOXNW-GZAMCDGTSA-N
Descrizione generale
Propamocarb-(propyl-d7) is an isotope-labeled analog of systemic fungicide propamocarb, wherein propyl protons are substituted by deuterium.
Applicazioni
Propamocarb-(propyl-d7) may be used as a deuterated internal standard to quantify the pesticide analytes in foods of plant origin by two-dimensional liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Isotope-labeled compounds are increasingly used in isotope dilution mass spectrometry (IDMS) for the quantitative analysis of pesticides. The major advantage of using this technique is that the isotope-labeled compounds have nearly the same physical properties as their non-labeled counterpart analogs, and thus show identical behavior during the workup and sample preparation process. This helps in overcoming the problems of matrix effects generally encountered in the usual LC-MS/GC-MS analysis potentially resulting in biased results. By spiking the sample before workup with its isotope labeled analog, the loss of analyte that leads to matrix effects can be determined and compensated.
Isotope-labeled compounds are increasingly used in isotope dilution mass spectrometry (IDMS) for the quantitative analysis of pesticides. The major advantage of using this technique is that the isotope-labeled compounds have nearly the same physical properties as their non-labeled counterpart analogs, and thus show identical behavior during the workup and sample preparation process. This helps in overcoming the problems of matrix effects generally encountered in the usual LC-MS/GC-MS analysis potentially resulting in biased results. By spiking the sample before workup with its isotope labeled analog, the loss of analyte that leads to matrix effects can be determined and compensated.
Note legali
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Simultaneous determination of pesticides, mycotoxins, tropane alkaloids, growth regulators, and pyrrolizidine alkaloids in oats and whole wheat grains after online clean-up via two-dimensional liquid chromatography tandem mass spectrometry
Urban M, et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 54(2), 98-111 (2019)
Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS/MS involving simultaneous extraction with methanol (QuPPe-method)
Anastassiades M, et al.
EU Reference Laboratie for Residue of Pesticides, 9, 1-60 (2015)
Simultaneous determination of pesticides, mycotoxins, and metabolites as well as other contaminants in cereals by LC-LC-MS/MS
Kresse M, et al.
Journal of Chromatography. B, Biomedical Applications, 1117(2), 86-102 (2019)
Quick method for the analysis of numerous highly polar pesticides in foods of plant origin via LC-MS/MS involving simultaneous extraction with methanol (QuPPe-method)
Anastassiades M, et al.
Chemosphere, 1117(2), 86-102 (2019)
Rosalía López-Ruiz et al.
Chemosphere, 226, 36-46 (2019-03-27)
In this study, fenamidone, propamocarb and their transformation products were monitored in cherry tomato, cucumber, and courgette samples. A mixture of both compounds, which have different physico-chemical characteristics, are commercially available (Consento®). For analysis, ultra high-performance liquid chromatography coupled to
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.