69484
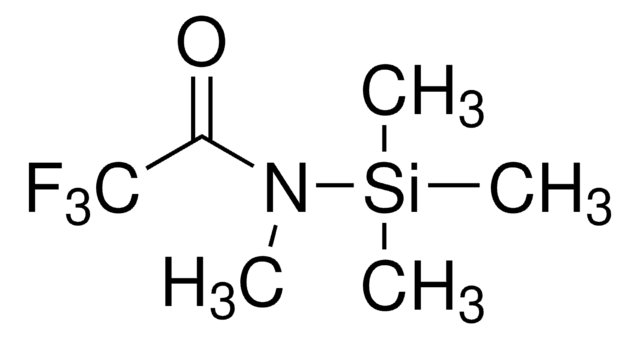
N-Methyl-N-trimethylsilylheptafluorobutyramide
for GC derivatization, LiChropur™, ≥90% (GC)
Sinonimo/i:
N-Trimethylsilyl-N-methylheptafluorobutyramide, MSHFBA
About This Item
Prodotti consigliati
Grado
for GC derivatization
Livello qualitativo
Saggio
≥90% (GC)
Forma fisica
liquid
Qualità
LiChropur™
Impiego in reazioni chimiche
reagent type: derivatization reagent
reaction type: Silylations
tecniche
gas chromatography (GC): suitable
Indice di rifrazione
n20/D 1.353 (lit.)
n20/D 1.353
P. eboll.
148 °C (lit.)
Densità
1.254 g/mL at 25 °C (lit.)
Stringa SMILE
CN(C(=O)C(F)(F)C(F)(F)C(F)(F)F)[Si](C)(C)C
InChI
1S/C8H12F7NOSi/c1-16(18(2,3)4)5(17)6(9,10)7(11,12)8(13,14)15/h1-4H3
CMXKINNDZCNCEI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Simultaneous quantitation of cocaine, opiates, and their metabolites in human hair by positive ion chemical ionization gas chromatography-mass spectrometry: This study demonstrates the application of N-Methyl-N-trimethylsilylheptafluorobutyramide in forensic toxicology to analyze drug residues in human hair, providing a robust method for detecting such compounds at trace levels (Höld KM, Wilkins DG, Rollins DE, Joseph RE Jr, Cone EJ, 1998).
- Detection of stanozolol in hair by negative ion chemical ionization mass spectrometry: The research utilizes N-Methyl-N-trimethylsilylheptafluorobutyramide for the sensitive detection of stanozolol, a performance-enhancing steroid, in hair samples. This method is particularly useful in sports doping analyses to ensure fair play (Höld KM, Wilkins DG, Crouch DJ, Rollins DE, Maes RA, 1996).
Altre note
Note legali
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
91.4 °F - closed cup
Punto d’infiammabilità (°C)
33 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.