52650
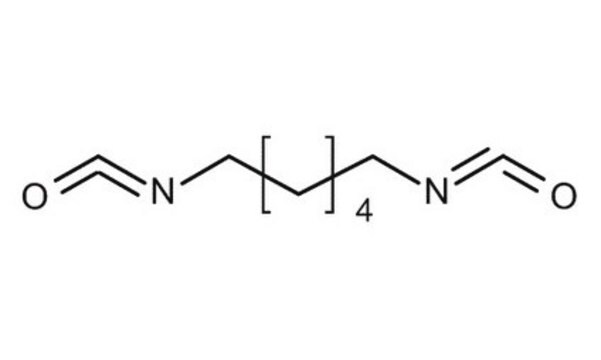
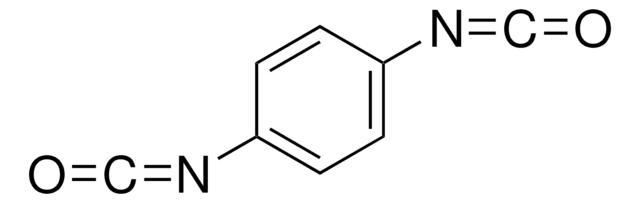
Hexamethylene diisocyanate
purum, ≥98.0% (GC)
Sinonimo/i:
1,6-Diisocyanatohexane
About This Item
Prodotti consigliati
Grado
purum
Livello qualitativo
Saggio
≥98.0% (GC)
Indice di rifrazione
n20/D 1.453
P. eboll.
82-85 °C/0.1 mmHg
Densità
1.047 g/mL at 20 °C (lit.)
Stringa SMILE
O=C=NCCCCCCN=C=O
InChI
1S/C8H12N2O2/c11-7-9-5-3-1-2-4-6-10-8-12/h1-6H2
RRAMGCGOFNQTLD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- A crosslinker to crosslink the polyurethane chains in the triblock copolymer gate dielectric, which is then deposited on the substrate to fabricate low-voltage organic thin-film transistors.
- A precursor in the preparation of electroactive shape memory polyurethane/graphene nanocomposites. These materials are usually used as actuators, sensors, artificial muscles, smart devices, and microswitches.
- A crosslinker in conjunction with Pluronic F127, a nonionic surfactant, to synthesize a poly(lactic acid) (PLA)-based hydrogel for biomedical applications.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 1 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
266.0 °F - Pensky-Martens closed cup
Punto d’infiammabilità (°C)
130 °C - Pensky-Martens closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
HPLC Analysis of Isocyanates on Titan™ C18
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.