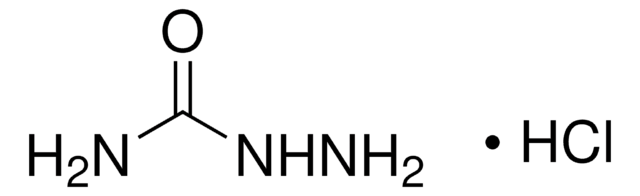
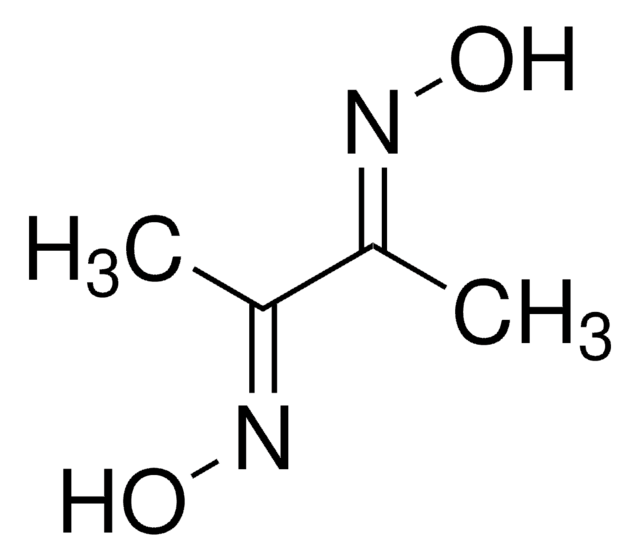
31550
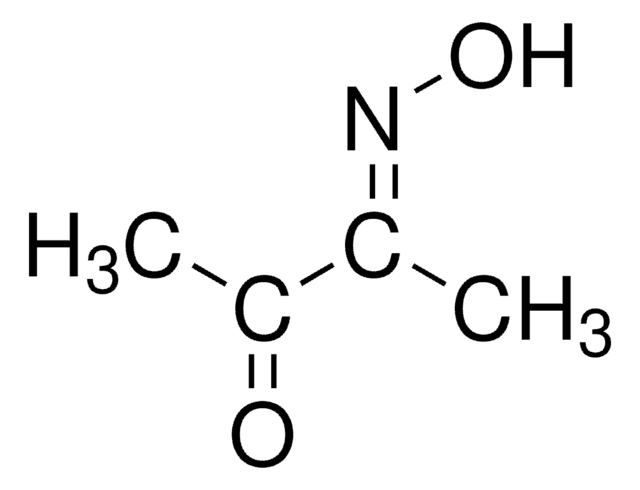
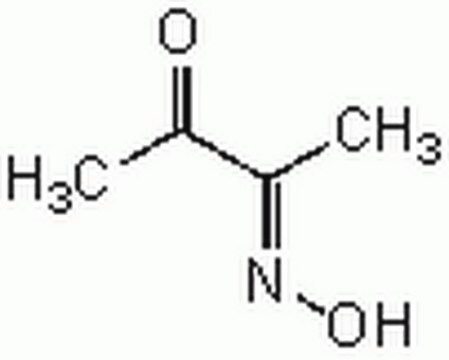
2,3-Butanedione monoxime
for spectrophotometric det. of urea, ≥99.0%
Sinonimo/i:
BDM
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3C(=NOH)COCH3
Numero CAS:
Peso molecolare:
101.10
Beilstein:
605582
Numero CE:
Numero MDL:
Codice UNSPSC:
41116105
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Livello qualitativo
Saggio
≥98.0% (N)
≥99.0%
Stato
solid
Qualità
for spectrophotometric det. of urea
tecniche
UV/Vis spectroscopy: suitable
Residuo alla calcinazione
≤0.05% (as SO4)
P. ebollizione
185-186 °C (lit.)
Punto di fusione
75-76 °C
75-78 °C (lit.)
Stringa SMILE
CC(=O)\C(C)=N\O
InChI
1S/C4H7NO2/c1-3(5-7)4(2)6/h7H,1-2H3/b5-3+
FSEUPUDHEBLWJY-HWKANZROSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2,3-Butanedione Monoxime (BDM) is also known as diacetyl monoxime, it is also a nucleophilic agent and it can dephosphorylates acetylcholinesterase poisoned with organophosphates. It is also a well-characterized, non-competitive inhibitor of skeletal muscle myosin-II, where it inhibits the chemical and motile activity.
Applicazioni
- A novel method to extend viability and functionality of living heart slices.: This research introduces a novel application of 2,3-Butanedione monoxime for prolonging the functional lifespan of cardiac tissue samples in experimental settings, offering insights into cardiac biology and potential therapeutic targets (Ross et al., 2023).
- Molecular Mechanisms of Deregulation of Muscle Contractility Caused by the R168H Mutation in TPM3 and Its Attenuation by Therapeutic Agents.: The study utilizes 2,3-Butanedione monoxime to investigate the molecular pathways affected by genetic mutations in muscle contractility, contributing to the understanding of muscle disorders and their management (Karpicheva et al., 2023).
- Generation of myocyte agonal Ca(2+) waves and contraction bands in perfused rat hearts following irreversible membrane permeabilisation.: Research employing 2,3-Butanedione monoxime investigates its role in inducing specific cellular events in cardiac cells under stress, highlighting its potential in studies of heart disease mechanisms and therapies (Morishita et al., 2023).
Risultati analitici
Solubility:
0.5 g are completely soluble and give a clear solution in 10 mL water or also in 10 mL ethanol.
Sensitivity test:
0.05 mg urea in 3 mL water and 5 mL conc. HCl together with a 3% solution in water of diacetylmonoxime heated on a water bath for 10 minutes give a light yellow color.
0.5 g are completely soluble and give a clear solution in 10 mL water or also in 10 mL ethanol.
Sensitivity test:
0.05 mg urea in 3 mL water and 5 mL conc. HCl together with a 3% solution in water of diacetylmonoxime heated on a water bath for 10 minutes give a light yellow color.
Altre note
Reagent for the colorimetric determination of urea and ureido-compounds; Spectrometric reagent for Co(II), Ni(II), Pd(II) and Re(VII); Reagent for the gravimetric determination of Ni(II)
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
W.B. Guenther
Analytical Letters, 12A, 1305-1305 (1979)
Improvements on the Prescott-Jones method for the colorimetric analysis of ureido compounds.
D B Shindler et al.
Analytical biochemistry, 97(2), 421-422 (1979-09-01)
Multiple Effects of 2, 3-Butanedione Monoxime.
Sellin LC and McArdle JJ/
Pharmacology & Toxicology, 74 (4-5), 305-313 (1994)
2, 3-Butanedione monoxime (BDM) as a myosin inhibitor.
Ostap EM.
Journal of Muscle Research and Cell Motility, 23 (4), 305-308 (2002)
Chang Liu et al.
Molecular plant, 11(11), 1389-1399 (2018-10-09)
The process of pollen germination is crucial for flowering plant reproduction, but the mechanisms through which pollen grains establish polarity and select germination sites are not well understood. In this study, we report that a formin family protein, AtFH5, is localized
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.